Association of AICD and Fe65 with Hirano bodies reduces transcriptional activation and initiation of apoptosis
- PMID: 20133016
- PMCID: PMC2894277
- DOI: 10.1016/j.neurobiolaging.2010.01.003
Association of AICD and Fe65 with Hirano bodies reduces transcriptional activation and initiation of apoptosis
Abstract
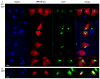
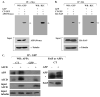
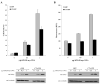
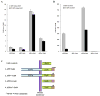
Hirano bodies are cytoplasmic inclusions predominantly found in the central nervous system associated with various conditions including aging and Alzheimer's disease (AD). Since most studies of Hirano bodies have been performed in post-mortem samples, the physiological roles of Hirano bodies have not been investigated. Astrocytoma H4 cells were employed to test the hypothesis that Hirano bodies interact with and modulate signaling by the C-terminal fragment of amyloid-β precursor protein (AICD). We demonstrated by immunofluorescence and immunoprecipitation that model Hirano bodies accumulate AICD. Since stimulation of transcription by AICD is dependent on its interaction with the nuclear adaptor protein Fe65, we examined localization of Fe65, and employed a dual luciferase reporter assay to test the effects of Hirano bodies on AICD- and Fe65-dependent modulation of gene expression. We find that both AICD and Fe65 are co-localized in model Hirano bodies. Model Hirano bodies also down-regulate both AICD-dependent apoptosis and AICD- and Fe65-dependent transcriptional activity. Thus, association of AICD and Fe65 with Hirano bodies impedes their function in promoting apoptosis and modulating transcription.
Copyright © 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure Statement: there are no conflicts of interest
Figures

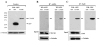
References
-
- Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. - PubMed
-
- Butterfield DA, Griffin S, Munch G, Pasinetti GM. Amyloid beta-peptide and amyloid pathology are central to the oxidative stress and inflammatory cascades under which Alzheimer's disease brain exists. J Alzheimer's Dis. 2002;4:193. - PubMed
-
- Cao X, Südhof TC. A transcriptionally active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001;293:115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

