Structure-based design of kinetic stabilizers that ameliorate the transthyretin amyloidoses
- PMID: 20133122
- PMCID: PMC2830738
- DOI: 10.1016/j.sbi.2009.12.009
Structure-based design of kinetic stabilizers that ameliorate the transthyretin amyloidoses
Abstract
Small molecules that bind to normally unoccupied thyroxine (T(4)) binding sites within transthyretin (TTR) in the blood stabilize the tetrameric ground state of TTR relative to the dissociative transition state and dramatically slow tetramer dissociation, the rate-limiting step for the process of amyloid fibril formation linked to neurodegeneration and cell death. These so-called TTR kinetic stabilizers have been designed using structure-based principles and one of these has recently been shown to halt the progression of a human TTR amyloid disease in a clinical trial, providing the first pharmacologic evidence that the process of amyloid fibril formation is causative. Structure-based design has now progressed to the point where highly selective, high affinity TTR kinetic stabilizers that lack undesirable off-target activities can be produced with high frequency.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures



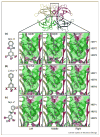
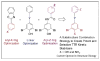
Similar articles
-
Native state kinetic stabilization as a strategy to ameliorate protein misfolding diseases: a focus on the transthyretin amyloidoses.Acc Chem Res. 2005 Dec;38(12):911-21. doi: 10.1021/ar020073i. Acc Chem Res. 2005. PMID: 16359163 Review.
-
Synthesis and characterization of potent bivalent amyloidosis inhibitors that bind prior to transthyretin tetramerization.J Am Chem Soc. 2003 Nov 5;125(44):13404-14. doi: 10.1021/ja030294z. J Am Chem Soc. 2003. PMID: 14583036
-
Semi-quantitative models for identifying potent and selective transthyretin amyloidogenesis inhibitors.Bioorg Med Chem Lett. 2017 Aug 1;27(15):3441-3449. doi: 10.1016/j.bmcl.2017.05.080. Epub 2017 May 26. Bioorg Med Chem Lett. 2017. PMID: 28625364 Free PMC article.
-
A substructure combination strategy to create potent and selective transthyretin kinetic stabilizers that prevent amyloidogenesis and cytotoxicity.J Am Chem Soc. 2010 Feb 3;132(4):1359-70. doi: 10.1021/ja908562q. J Am Chem Soc. 2010. PMID: 20043671 Free PMC article.
-
Nearly 200 X-ray crystal structures of transthyretin: what do they tell us about this protein and the design of drugs for TTR amyloidoses?Curr Med Chem. 2012;19(15):2324-42. doi: 10.2174/092986712800269335. Curr Med Chem. 2012. PMID: 22471981 Review.
Cited by
-
The flavonoid luteolin, but not luteolin-7-O-glucoside, prevents a transthyretin mediated toxic response.PLoS One. 2015 May 28;10(5):e0128222. doi: 10.1371/journal.pone.0128222. eCollection 2015. PLoS One. 2015. PMID: 26020516 Free PMC article.
-
A fluorogenic aryl fluorosulfate for intraorganellar transthyretin imaging in living cells and in Caenorhabditis elegans.J Am Chem Soc. 2015 Jun 17;137(23):7404-14. doi: 10.1021/jacs.5b03042. Epub 2015 Jun 8. J Am Chem Soc. 2015. PMID: 26051248 Free PMC article.
-
Drug Discovery and Development in Rare Diseases: Taking a Closer Look at the Tafamidis Story.Drug Des Devel Ther. 2021 Mar 18;15:1225-1243. doi: 10.2147/DDDT.S289772. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 33776421 Free PMC article. Review.
-
Deciphering a molecular mechanism of neonatal diabetes mellitus by the chemical synthesis of a protein diastereomer, [D-AlaB8]human proinsulin.J Biol Chem. 2014 Aug 22;289(34):23683-92. doi: 10.1074/jbc.M114.572040. Epub 2014 Jul 7. J Biol Chem. 2014. PMID: 25002580 Free PMC article.
-
Conformational and aggregation properties of a PEGylated alanine-rich polypeptide.Biomacromolecules. 2011 Jun 13;12(6):2184-92. doi: 10.1021/bm200272w. Epub 2011 May 9. Biomacromolecules. 2011. PMID: 21553871 Free PMC article.
References
-
- Colon W, Kelly JW. Partial denaturation of transthyretin is sufficient for amyloid fibril formation in vitro. Biochemistry. 1992;31:8654–8660. - PubMed
-
- Kelly JW, Colon W, Lai Z, Lashuel HA, McCulloch J, McCutchen SL, Miroy GJ, Peterson SA. Transthyretin quaternary and tertiary structural changes facilitate misassembly into amyloid. Adv Protein Chem. 1997;50:161–181. - PubMed
-
- Johnson SM, Wiseman RL, Sekijima Y, Green NS, Adamski-Werner SL, Kelly JW. Native state kinetic stabilization as a strategy to ameliorate protein misfolding diseases: a focus on the transthyretin amyloidoses. Acc Chem Res. 2005;38:911–921. - PubMed
-
Review summarizing why ligand binding causes stabilization of the native state over the dissociative transition state raising the kinetic barrier for dissociation and, thus preventing amyloidogenesis.
-
- Walsh DM, Selkoe DJ. Abeta oligomers– a decade of discovery. J Neurochem. 2007;101:1172–1184. - PubMed
-
- Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT., Jr Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418:291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

