Structure-based design of kinetic stabilizers that ameliorate the transthyretin amyloidoses
- PMID: 20133122
- PMCID: PMC2830738
- DOI: 10.1016/j.sbi.2009.12.009
Structure-based design of kinetic stabilizers that ameliorate the transthyretin amyloidoses
Abstract
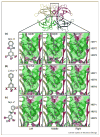
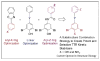
Small molecules that bind to normally unoccupied thyroxine (T(4)) binding sites within transthyretin (TTR) in the blood stabilize the tetrameric ground state of TTR relative to the dissociative transition state and dramatically slow tetramer dissociation, the rate-limiting step for the process of amyloid fibril formation linked to neurodegeneration and cell death. These so-called TTR kinetic stabilizers have been designed using structure-based principles and one of these has recently been shown to halt the progression of a human TTR amyloid disease in a clinical trial, providing the first pharmacologic evidence that the process of amyloid fibril formation is causative. Structure-based design has now progressed to the point where highly selective, high affinity TTR kinetic stabilizers that lack undesirable off-target activities can be produced with high frequency.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures



References
-
- Colon W, Kelly JW. Partial denaturation of transthyretin is sufficient for amyloid fibril formation in vitro. Biochemistry. 1992;31:8654–8660. - PubMed
-
- Kelly JW, Colon W, Lai Z, Lashuel HA, McCulloch J, McCutchen SL, Miroy GJ, Peterson SA. Transthyretin quaternary and tertiary structural changes facilitate misassembly into amyloid. Adv Protein Chem. 1997;50:161–181. - PubMed
-
- Johnson SM, Wiseman RL, Sekijima Y, Green NS, Adamski-Werner SL, Kelly JW. Native state kinetic stabilization as a strategy to ameliorate protein misfolding diseases: a focus on the transthyretin amyloidoses. Acc Chem Res. 2005;38:911–921. - PubMed
-
Review summarizing why ligand binding causes stabilization of the native state over the dissociative transition state raising the kinetic barrier for dissociation and, thus preventing amyloidogenesis.
-
- Walsh DM, Selkoe DJ. Abeta oligomers– a decade of discovery. J Neurochem. 2007;101:1172–1184. - PubMed
-
- Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT., Jr Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418:291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

