ADF/cofilin: a functional node in cell biology
- PMID: 20133134
- PMCID: PMC2849908
- DOI: 10.1016/j.tcb.2010.01.001
ADF/cofilin: a functional node in cell biology
Abstract
Recent findings have significantly expanded our understanding of the regulation of actin-depolymerizing factor (ADF)/cofilin proteins and the profound multifaceted impact that these well-established regulators of actin dynamics have on cell biology. In this review we discuss new aspects of previously documented regulation, such as phosphorylation, but also cover novel recently established modes of regulation and functions of ADF (also known as destrin)/cofilin. We now understand that their activity responds to a vast array of inputs far greater than previously appreciated and that these proteins not only feed back to the crucially important dynamics of actin, but also to apoptosis cascades, phospholipid metabolism, and gene expression. We argue that this ability to respond to physiological changes by modulating those same changes makes the ADF/cofilin protein family a homeostatic regulator or 'functional node' in cell biology.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
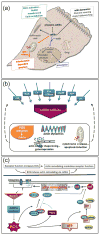
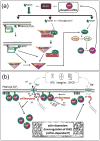
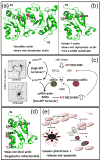
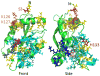
References
-
- Gurniak CB, et al. The actin depolymerizing factor n-cofilin is essential for neural tube morphogenesis and neural crest cell migration. Dev Biol. 2005;278:231–241. - PubMed
-
- Ikeda S, et al. Aberrant actin cytoskeleton leads to accelerated proliferation of corneal epithelial cells in mice deficient for destrin (actin depolymerizing factor) Hum Mol Genet. 2003;12:1029–1036. - PubMed
-
- Van Troys M, et al. Ins and outs of ADF/cofilin activity and regulation. Eur J Cell Biol. 2008;87:649–667. - PubMed
-
- Andrianantoandro E, Pollard TD. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol Cell. 2006;24:13–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

