Interaction fidelity in two-component signaling
- PMID: 20133181
- PMCID: PMC2847666
- DOI: 10.1016/j.mib.2010.01.007
Interaction fidelity in two-component signaling
Abstract
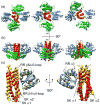
Two component signal transduction systems and phosphorelays have been adapted and amplified by bacteria to respond to a multitude of environmental, metabolic and cell cycle signals while maintaining essentially identical structures for the domains responsible for recognition and phosphotransfer between the sensor histidine kinase and the response regulator. Co-crystal structures of these domains have revealed the variable residues at the interaction surface of the two components responsible for interaction specificity in signal transfer. This information has formed the basis for the development and validation of statistical methods to identify interaction residues and surfaces from compiled databases of interacting proteins and holds forth the promise of determining structures of multi-protein complexes and signaling networks.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Using structural information to change the phosphotransfer specificity of a two-component chemotaxis signalling complex.PLoS Biol. 2010 Feb 9;8(2):e1000306. doi: 10.1371/journal.pbio.1000306. PLoS Biol. 2010. PMID: 20161720 Free PMC article.
-
A transient interaction between two phosphorelay proteins trapped in a crystal lattice reveals the mechanism of molecular recognition and phosphotransfer in signal transduction.Structure. 2000 Aug 15;8(8):851-62. doi: 10.1016/s0969-2126(00)00174-x. Structure. 2000. PMID: 10997904
-
Summary of useful methods for two-component system research.Curr Opin Microbiol. 2010 Apr;13(2):246-52. doi: 10.1016/j.mib.2010.01.006. Curr Opin Microbiol. 2010. PMID: 20138001 Review.
-
SpaK/SpaR two-component system characterized by a structure-driven domain-fusion method and in vitro phosphorylation studies.PLoS Comput Biol. 2009 Jun;5(6):e1000401. doi: 10.1371/journal.pcbi.1000401. Epub 2009 Jun 5. PLoS Comput Biol. 2009. PMID: 19503843 Free PMC article.
-
Evolution of signalling in the sporulation phosphorelay.Mol Microbiol. 2002 Oct;46(2):297-304. doi: 10.1046/j.1365-2958.2002.03186.x. Mol Microbiol. 2002. PMID: 12406209 Review.
Cited by
-
From photon to signal in phytochromes: similarities and differences between prokaryotic and plant phytochromes.J Plant Res. 2016 Mar;129(2):123-35. doi: 10.1007/s10265-016-0789-0. Epub 2016 Jan 27. J Plant Res. 2016. PMID: 26818948 Review.
-
Interactions of the CpxA sensor kinase and cognate CpxR response regulator from Yersinia pseudotuberculosis.BMC Res Notes. 2012 Sep 27;5:536. doi: 10.1186/1756-0500-5-536. BMC Res Notes. 2012. PMID: 23013530 Free PMC article.
-
A Variable Active Site Residue Influences the Kinetics of Response Regulator Phosphorylation and Dephosphorylation.Biochemistry. 2016 Oct 4;55(39):5595-5609. doi: 10.1021/acs.biochem.6b00645. Epub 2016 Sep 19. Biochemistry. 2016. PMID: 27589219 Free PMC article.
-
Two-component signaling circuit structure and properties.Curr Opin Microbiol. 2010 Apr;13(2):184-9. doi: 10.1016/j.mib.2010.01.009. Epub 2010 Feb 10. Curr Opin Microbiol. 2010. PMID: 20149717 Free PMC article. Review.
-
Single cell super-resolution imaging of E. coli OmpR during environmental stress.Integr Biol (Camb). 2015 Oct;7(10):1297-308. doi: 10.1039/c5ib00077g. Epub 2015 Jul 9. Integr Biol (Camb). 2015. PMID: 26156621 Free PMC article.
References
-
- Hoch JA, Silhavy TJ. Two-component signal transduction. Washington, D.C: ASM Press; 1995.
-
- Zapf J, Sen U, Madhusudan Hoch JA, Varughese KI. A transient interaction between two phosphorelay proteins trapped in a crystal lattice reveals the mechanism of molecular recognition and phosphotransfer in signal transduction. Structure. 2000;8:851–862. This manuscript presents the first high-resolution insight into the SK/RR interaction specificity in the form of the Spo0B/Spo0F co-crystal structure. Although Spo0B is a phosphotransfer protein in the sporulation phosphorelay rather than a SK, the structure demonstrates that it shares fold and function with the SK. As such, this manuscript proved to be the major milestone in the understanding of signaling fidelity by revealing the contact interface between SK and RR. - PubMed
-
- Stock AM, Mottonen JM, Stock JB, Schutt CE. Three-dimensional structure of CheY, the response regulator of bacterial chemotaxis. Nature. 1989;337:745–749. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

