Interaction fidelity in two-component signaling
- PMID: 20133181
- PMCID: PMC2847666
- DOI: 10.1016/j.mib.2010.01.007
Interaction fidelity in two-component signaling
Abstract
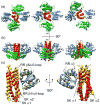
Two component signal transduction systems and phosphorelays have been adapted and amplified by bacteria to respond to a multitude of environmental, metabolic and cell cycle signals while maintaining essentially identical structures for the domains responsible for recognition and phosphotransfer between the sensor histidine kinase and the response regulator. Co-crystal structures of these domains have revealed the variable residues at the interaction surface of the two components responsible for interaction specificity in signal transfer. This information has formed the basis for the development and validation of statistical methods to identify interaction residues and surfaces from compiled databases of interacting proteins and holds forth the promise of determining structures of multi-protein complexes and signaling networks.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures

References
-
- Hoch JA, Silhavy TJ. Two-component signal transduction. Washington, D.C: ASM Press; 1995.
-
- Zapf J, Sen U, Madhusudan Hoch JA, Varughese KI. A transient interaction between two phosphorelay proteins trapped in a crystal lattice reveals the mechanism of molecular recognition and phosphotransfer in signal transduction. Structure. 2000;8:851–862. This manuscript presents the first high-resolution insight into the SK/RR interaction specificity in the form of the Spo0B/Spo0F co-crystal structure. Although Spo0B is a phosphotransfer protein in the sporulation phosphorelay rather than a SK, the structure demonstrates that it shares fold and function with the SK. As such, this manuscript proved to be the major milestone in the understanding of signaling fidelity by revealing the contact interface between SK and RR. - PubMed
-
- Stock AM, Mottonen JM, Stock JB, Schutt CE. Three-dimensional structure of CheY, the response regulator of bacterial chemotaxis. Nature. 1989;337:745–749. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

