Long-term impact of efavirenz on neuropsychological performance and symptoms in HIV-infected individuals (ACTG 5097s)
- PMID: 20133265
- PMCID: PMC2958039
- DOI: 10.1310/hct1006-343
Long-term impact of efavirenz on neuropsychological performance and symptoms in HIV-infected individuals (ACTG 5097s)
Abstract
Background: Efavirenz (EFV) is an antiretroviral (ARV) drug associated with neuropsychological effects. Limited data describing the long-term impact of EFV-based regimens on neuropsychological performance over more than 3 years are available.
Methods: We enrolled a subset of participants from a large initially EFV placebo-controlled trial of therapies for HIV subjects naïve to ARV treatment (A5095). Clinical follow-up continued for 184 weeks of study. Subjects were assessed with brief neuropsychological testing, a symptom questionnaire of EFV-associated symptoms, the Pittsburgh Sleep Index, Center for Epidemiologic Studies-Depression Scale, and an anxiety rating interview.
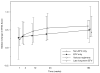
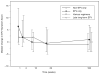
Results: Over 184 weeks on EFV, the median NPZ3 score in 86 evaluable patients improved from baseline by +0.5 (p < .01); all components improved, although higher EFV levels were associated with slightly lower responses. Overall symptom scores did not change, while EFV-associated CNS symptoms increased (p = .01). Median change of bad dream sleep scores and anxiety increased from the baseline while global depression score decreased.
Conclusions: In participants who continued EFV-based regimens, neuropsychological performance improvement from baseline was maintained over 3 years. EFV-based treatment was generally well tolerated, but small increases from baseline in EFV-associated symptoms, bad dreams, and anxiety were detected.
Trial registration: ClinicalTrials.gov NCT00013529.
Figures
Similar articles
-
Neuropsychological changes in efavirenz switch regimens.AIDS. 2019 Jul 1;33(8):1307-1314. doi: 10.1097/QAD.0000000000002206. AIDS. 2019. PMID: 30932965 Free PMC article.
-
Pharmacodynamics of efavirenz 400 mg in treatment-naïve Chinese HIV-infected patients in a prospective cohort study.BMC Infect Dis. 2021 Jan 23;21(1):112. doi: 10.1186/s12879-021-05802-8. BMC Infect Dis. 2021. PMID: 33485301 Free PMC article.
-
Tolerability of central nervous system symptoms among HIV-1 infected efavirenz users: analysis of patient electronic medical record data.AIDS Care. 2017 Aug;29(8):1067-1073. doi: 10.1080/09540121.2017.1280123. Epub 2017 Feb 1. AIDS Care. 2017. PMID: 28147708
-
Efavirenz in the therapy of HIV infection.Expert Opin Drug Metab Toxicol. 2010 Jan;6(1):95-103. doi: 10.1517/17425250903483207. Expert Opin Drug Metab Toxicol. 2010. PMID: 20001610 Free PMC article. Review.
-
Adverse Neuropsychiatric Events and Recreational Use of Efavirenz and Other HIV-1 Antiretroviral Drugs.Pharmacol Rev. 2018 Jul;70(3):684-711. doi: 10.1124/pr.117.013706. Pharmacol Rev. 2018. PMID: 29945900 Review.
Cited by
-
Application of the Chinese Version of the Pittsburgh Sleep Quality Index in People Living With HIV: Preliminary Reliability and Validity.Front Psychiatry. 2021 Jul 6;12:676022. doi: 10.3389/fpsyt.2021.676022. eCollection 2021. Front Psychiatry. 2021. PMID: 34295273 Free PMC article.
-
Oral Administration of Efavirenz Dysregulates the Tph2 Gene in Brain Serotonergic Areas and Alters Weight and Mood in Mice.Pharmaceuticals (Basel). 2024 Jun 18;17(6):801. doi: 10.3390/ph17060801. Pharmaceuticals (Basel). 2024. PMID: 38931468 Free PMC article.
-
Neuropsychological changes in efavirenz switch regimens.AIDS. 2019 Jul 1;33(8):1307-1314. doi: 10.1097/QAD.0000000000002206. AIDS. 2019. PMID: 30932965 Free PMC article.
-
Incidence of neuropsychiatric side effects of efavirenz in HIV-positive treatment-naïve patients in public-sector clinics in the Eastern Cape.South Afr J HIV Med. 2016 Jun 30;17(1):452. doi: 10.4102/sajhivmed.v17i1.452. eCollection 2016. South Afr J HIV Med. 2016. PMID: 29568611 Free PMC article.
-
PharmGKB summary: Efavirenz pathway, pharmacokinetics.Pharmacogenet Genomics. 2015 Jul;25(7):363-76. doi: 10.1097/FPC.0000000000000145. Pharmacogenet Genomics. 2015. PMID: 25966836 Free PMC article. Review. No abstract available.
References
-
- Staszewski S, Morales-Ramirez J, Tashima K, et al. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. New Engl J Med. 1999;341:1865–1873. - PubMed
-
- Hammer SM, Eron JJ, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society – USA Panel. JAMA. 2008;300(5):555–570. - PubMed
-
- Bristol-Myers Squibb; PDR net®. Sustiva® (efavirenz) package insert. 2008.
-
- Clifford DB, Evans S, Yang Y, et al. Impact on efavirenz on neuropsychological performance and symptoms in HIV-infected individuals. Ann Intern Med. 2005;143:714–721. - PubMed
-
- Gulick RM, Ribaudo HJ, Shikuma CM, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med. 2004;350:1850–1861. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- AI68634/AI/NIAID NIH HHS/United States
- U01 AI027661/AI/NIAID NIH HHS/United States
- UL1 RR024996/RR/NCRR NIH HHS/United States
- P30 AI054907/AI/NIAID NIH HHS/United States
- U01 NS032228/NS/NINDS NIH HHS/United States
- U01 AI069450/AI/NIAID NIH HHS/United States
- UM1 AI069474/AI/NIAID NIH HHS/United States
- U01 AI069511/AI/NIAID NIH HHS/United States
- UM1 AI069532/AI/NIAID NIH HHS/United States
- UM1 AI069419/AI/NIAID NIH HHS/United States
- R01 NS032228/NS/NINDS NIH HHS/United States
- U01 AI069419/AI/NIAID NIH HHS/United States
- U01 AI069474/AI/NIAID NIH HHS/United States
- U01 AI069423/AI/NIAID NIH HHS/United States
- AI51966/AI/NIAID NIH HHS/United States
- UM1 AI069472/AI/NIAID NIH HHS/United States
- U01 AI069467/AI/NIAID NIH HHS/United States
- M01 RR000096/RR/NCRR NIH HHS/United States
- M01 RR000046/RR/NCRR NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- UM1 AI069495/AI/NIAID NIH HHS/United States
- AI38858/AI/NIAID NIH HHS/United States
- UM1 AI069471/AI/NIAID NIH HHS/United States
- U01 AI069439/AI/NIAID NIH HHS/United States
- U01 AI069532/AI/NIAID NIH HHS/United States
- K24 AI051966/AI/NIAID NIH HHS/United States
- UM1 AI069439/AI/NIAID NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- R01 AI058740/AI/NIAID NIH HHS/United States
- AI069495/AI/NIAID NIH HHS/United States
- U01 AI069432/AI/NIAID NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- U01 AI046370/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- U01 AI069415/AI/NIAID NIH HHS/United States
- UL1 RR024160/RR/NCRR NIH HHS/United States
- U01 AI069495/AI/NIAID NIH HHS/United States
- U01 AI025859/AI/NIAID NIH HHS/United States
- UM1 AI069450/AI/NIAID NIH HHS/United States
- UM1 AI069511/AI/NIAID NIH HHS/United States
- U01 AI069494/AI/NIAID NIH HHS/United States
- U01AI068636/AI/NIAID NIH HHS/United States
- RR024996/RR/NCRR NIH HHS/United States
- U01 AI069471/AI/NIAID NIH HHS/United States
- NS032228/NS/NINDS NIH HHS/United States
- U01 AI069472/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- UL1 RR024979/RR/NCRR NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials