Long-term impact of efavirenz on neuropsychological performance and symptoms in HIV-infected individuals (ACTG 5097s)
- PMID: 20133265
- PMCID: PMC2958039
- DOI: 10.1310/hct1006-343
Long-term impact of efavirenz on neuropsychological performance and symptoms in HIV-infected individuals (ACTG 5097s)
Abstract
Background: Efavirenz (EFV) is an antiretroviral (ARV) drug associated with neuropsychological effects. Limited data describing the long-term impact of EFV-based regimens on neuropsychological performance over more than 3 years are available.
Methods: We enrolled a subset of participants from a large initially EFV placebo-controlled trial of therapies for HIV subjects naïve to ARV treatment (A5095). Clinical follow-up continued for 184 weeks of study. Subjects were assessed with brief neuropsychological testing, a symptom questionnaire of EFV-associated symptoms, the Pittsburgh Sleep Index, Center for Epidemiologic Studies-Depression Scale, and an anxiety rating interview.
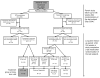
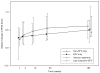
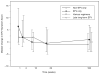
Results: Over 184 weeks on EFV, the median NPZ3 score in 86 evaluable patients improved from baseline by +0.5 (p < .01); all components improved, although higher EFV levels were associated with slightly lower responses. Overall symptom scores did not change, while EFV-associated CNS symptoms increased (p = .01). Median change of bad dream sleep scores and anxiety increased from the baseline while global depression score decreased.
Conclusions: In participants who continued EFV-based regimens, neuropsychological performance improvement from baseline was maintained over 3 years. EFV-based treatment was generally well tolerated, but small increases from baseline in EFV-associated symptoms, bad dreams, and anxiety were detected.
Trial registration: ClinicalTrials.gov NCT00013529.
Figures
References
-
- Staszewski S, Morales-Ramirez J, Tashima K, et al. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. New Engl J Med. 1999;341:1865–1873. - PubMed
-
- Hammer SM, Eron JJ, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society – USA Panel. JAMA. 2008;300(5):555–570. - PubMed
-
- Bristol-Myers Squibb; PDR net®. Sustiva® (efavirenz) package insert. 2008.
-
- Clifford DB, Evans S, Yang Y, et al. Impact on efavirenz on neuropsychological performance and symptoms in HIV-infected individuals. Ann Intern Med. 2005;143:714–721. - PubMed
-
- Gulick RM, Ribaudo HJ, Shikuma CM, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med. 2004;350:1850–1861. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- AI68634/AI/NIAID NIH HHS/United States
- U01 AI027661/AI/NIAID NIH HHS/United States
- UL1 RR024996/RR/NCRR NIH HHS/United States
- P30 AI054907/AI/NIAID NIH HHS/United States
- U01 NS032228/NS/NINDS NIH HHS/United States
- U01 AI069450/AI/NIAID NIH HHS/United States
- UM1 AI069474/AI/NIAID NIH HHS/United States
- U01 AI069511/AI/NIAID NIH HHS/United States
- UM1 AI069532/AI/NIAID NIH HHS/United States
- UM1 AI069419/AI/NIAID NIH HHS/United States
- R01 NS032228/NS/NINDS NIH HHS/United States
- U01 AI069419/AI/NIAID NIH HHS/United States
- U01 AI069474/AI/NIAID NIH HHS/United States
- U01 AI069423/AI/NIAID NIH HHS/United States
- AI51966/AI/NIAID NIH HHS/United States
- UM1 AI069472/AI/NIAID NIH HHS/United States
- U01 AI069467/AI/NIAID NIH HHS/United States
- M01 RR000096/RR/NCRR NIH HHS/United States
- M01 RR000046/RR/NCRR NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- UM1 AI069495/AI/NIAID NIH HHS/United States
- AI38858/AI/NIAID NIH HHS/United States
- UM1 AI069471/AI/NIAID NIH HHS/United States
- U01 AI069439/AI/NIAID NIH HHS/United States
- U01 AI069532/AI/NIAID NIH HHS/United States
- K24 AI051966/AI/NIAID NIH HHS/United States
- UM1 AI069439/AI/NIAID NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- R01 AI058740/AI/NIAID NIH HHS/United States
- AI069495/AI/NIAID NIH HHS/United States
- U01 AI069432/AI/NIAID NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- U01 AI046370/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- U01 AI069415/AI/NIAID NIH HHS/United States
- UL1 RR024160/RR/NCRR NIH HHS/United States
- U01 AI069495/AI/NIAID NIH HHS/United States
- U01 AI025859/AI/NIAID NIH HHS/United States
- UM1 AI069450/AI/NIAID NIH HHS/United States
- UM1 AI069511/AI/NIAID NIH HHS/United States
- U01 AI069494/AI/NIAID NIH HHS/United States
- U01AI068636/AI/NIAID NIH HHS/United States
- RR024996/RR/NCRR NIH HHS/United States
- U01 AI069471/AI/NIAID NIH HHS/United States
- NS032228/NS/NINDS NIH HHS/United States
- U01 AI069472/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- UL1 RR024979/RR/NCRR NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials