Protective and damaging effects of platelets in acute cholestatic liver injury revealed by depletion and inhibition strategies
- PMID: 20133375
- PMCID: PMC2855361
- DOI: 10.1093/toxsci/kfq042
Protective and damaging effects of platelets in acute cholestatic liver injury revealed by depletion and inhibition strategies
Abstract
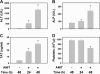
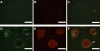
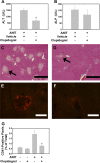
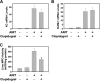
Alpha-naphthylisothiocyanate (ANIT) causes cholestatic hepatitis characterized by intrahepatic bile duct epithelial cell injury and periportal hepatocellular necrosis. The progression of ANIT-induced hepatocyte injury is reported to involve extrahepatic cells including platelets. We showed recently that the procoagulant protein tissue factor (TF) is essential for ANIT-induced coagulation and contributes to ANIT-induced liver necrosis. Platelets have been shown to express TF and can contribute to coagulation cascade activation. To this end, we tested the hypothesis that platelet-dependent coagulation contributes to ANIT-induced liver injury. In ANIT (60 mg/kg)-treated mice, activation of the coagulation cascade occurred prior to a decrease of platelets in the blood. Immunostaining for glycoprotein IIb (CD41) revealed platelet accumulation along the borders of necrotic foci in livers of ANIT-treated mice. Antibody-mediated platelet depletion did not affect coagulation but markedly affected liver histopathology in ANIT-treated mice. Platelet depletion induced marked pooling of blood within necrotic lesions consistent with parenchymal-type peliosis as early as 24 h after ANIT treatment. In contrast, treatment with the P2Y(12) inhibitor clopidogrel significantly reduced ANIT-induced hepatocyte necrosis and serum alanine aminotransferase activity but did not exaggerate bleeding into necrotic foci. Clopidogrel also reduced hepatic neutrophil accumulation but did not affect induction of Intercellular adhesion molecule-1 or chemokine CxC motif ligand-1 messenger RNA expression in liver. The data indicate that ANIT-induced coagulation is platelet independent and that platelets contribute to ANIT-induced hepatocyte necrosis by promoting neutrophil accumulation. In contrast, severe thrombocytopenia induces parenchymal-type peliosis in the livers of ANIT-treated mice, a rare hepatic lesion associated with pooling of blood in the liver.
Figures


Similar articles
-
Tissue factor-dependent coagulation contributes to alpha-naphthylisothiocyanate-induced cholestatic liver injury in mice.Am J Physiol Gastrointest Liver Physiol. 2009 Apr;296(4):G840-9. doi: 10.1152/ajpgi.90639.2008. Epub 2009 Jan 29. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19179621 Free PMC article.
-
Tissue factor contributes to neutrophil CD11b expression in alpha-naphthylisothiocyanate-treated mice.Toxicol Appl Pharmacol. 2011 Feb 1;250(3):256-62. doi: 10.1016/j.taap.2010.11.002. Epub 2010 Nov 9. Toxicol Appl Pharmacol. 2011. PMID: 21070799 Free PMC article.
-
Inhibition of PAR-4 and P2Y12 receptor-mediated platelet activation produces distinct hepatic pathologies in experimental xenobiotic-induced cholestatic liver disease.Toxicology. 2016 Jul 15;365:9-16. doi: 10.1016/j.tox.2016.07.021. Epub 2016 Jul 27. Toxicology. 2016. PMID: 27475285 Free PMC article.
-
Coagulation in liver toxicity and disease: role of hepatocyte tissue factor.Thromb Res. 2014 May;133 Suppl 1(0 1):S57-9. doi: 10.1016/j.thromres.2014.03.023. Thromb Res. 2014. PMID: 24759146 Free PMC article. Review.
-
The Provocative Roles of Platelets in Liver Disease and Cancer.Front Oncol. 2021 Jul 21;11:643815. doi: 10.3389/fonc.2021.643815. eCollection 2021. Front Oncol. 2021. PMID: 34367949 Free PMC article. Review.
Cited by
-
Coagulation-driven platelet activation reduces cholestatic liver injury and fibrosis in mice.J Thromb Haemost. 2015 Jan;13(1):57-71. doi: 10.1111/jth.12770. Epub 2014 Dec 11. J Thromb Haemost. 2015. PMID: 25353084 Free PMC article.
-
Platelets are relevant mediators of renal injury induced by primary endothelial lesions.Am J Physiol Renal Physiol. 2015 Jun 1;308(11):F1238-46. doi: 10.1152/ajprenal.00535.2014. Epub 2015 Apr 1. Am J Physiol Renal Physiol. 2015. PMID: 25834071 Free PMC article.
-
Activation of CD40 with platelet derived CD154 promotes reactive oxygen species dependent death of human hepatocytes during hypoxia and reoxygenation.PLoS One. 2012;7(1):e30867. doi: 10.1371/journal.pone.0030867. Epub 2012 Jan 25. PLoS One. 2012. PMID: 22295117 Free PMC article.
-
Platelets and protease-activated receptor-4 contribute to acetaminophen-induced liver injury in mice.Blood. 2015 Oct 8;126(15):1835-43. doi: 10.1182/blood-2014-09-598656. Epub 2015 Jul 15. Blood. 2015. PMID: 26179083 Free PMC article.
-
Platelet-neutrophil interactions as drivers of inflammatory and thrombotic disease.Cell Tissue Res. 2018 Mar;371(3):567-576. doi: 10.1007/s00441-017-2727-4. Epub 2017 Nov 25. Cell Tissue Res. 2018. PMID: 29178039 Free PMC article. Review.
References
-
- Abdel-Salam OM, Baiuomy AR, Ameen A, Hassan NS. A study of unfractionated and low molecular weight heparins in a model of cholestatic liver injury in the rat. Pharmacol. Res. 2005;51(1):59–67. - PubMed
-
- Bailie MB, Pearson JM, Lappin PB, Killam AL, Roth RA. Platelets and alpha-naphthylisothiocyanate-induced liver injury. Toxicol. Appl. Pharmacol. 1994;129(2):207–213. - PubMed
-
- Becker BA, Plaa GL. The nature of alpha-naphthylisothiocyanate-induced cholestasis. Toxicol. Appl. Pharmacol. 1965;7(5):680–685. - PubMed
-
- Belovejdov V, Staribratova D, Dikov D. Microscopic peliosis of pancreatic islets associated with thrombotic thrombocytopenic purpura. Pathol. Oncol. Res. 2008;14(2):205–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

