Oxidation of the endogenous cannabinoid arachidonoyl ethanolamide by the cytochrome P450 monooxygenases: physiological and pharmacological implications
- PMID: 20133390
- PMCID: PMC2835397
- DOI: 10.1124/pr.109.001081
Oxidation of the endogenous cannabinoid arachidonoyl ethanolamide by the cytochrome P450 monooxygenases: physiological and pharmacological implications
Abstract
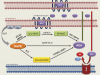
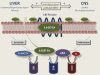
Arachidonoyl ethanolamide (anandamide) is an endogenous amide of arachidonic acid and an important signaling mediator of the endocannabinoid system. Given its numerous roles in maintaining normal physiological function and modulating pathophysiological responses throughout the body, the endocannabinoid system is an important pharmacological target amenable to manipulation directly by cannabinoid receptor ligands or indirectly by drugs that alter endocannabinoid synthesis and inactivation. The latter approach has the possible advantage of more selectivity, thus there is the potential for fewer untoward effects like those that are traditionally associated with cannabinoid receptor ligands. In that regard, inhibitors of the principal inactivating enzyme for anandamide, fatty acid amide hydrolase (FAAH), are currently in development for the treatment of pain and inflammation. However, several pathways involved in anandamide synthesis, metabolism, and inactivation all need to be taken into account when evaluating the effects of FAAH inhibitors and similar agents in preclinical models and assessing their clinical potential. Anandamide undergoes oxidation by several human cytochrome P450 (P450) enzymes, including CYP3A4, CYP4F2, CYP4X1, and the highly polymorphic CYP2D6, forming numerous structurally diverse lipids, which are likely to have important physiological roles, as evidenced by the demonstration that a P450-derived epoxide of anandamide is a potent agonist for the cannabinoid receptor 2. The focus of this review is to emphasize the need for a better understanding of the P450-mediated pathways of the metabolism of anandamide, because these are likely to be important in mediating endocannabinoid signaling as well as the pharmacological responses to endocannabinoid-targeting drugs.
Figures

Similar articles
-
Anandamide oxidation by wild-type and polymorphically expressed CYP2B6 and CYP2D6.Drug Metab Dispos. 2011 May;39(5):782-8. doi: 10.1124/dmd.110.036707. Epub 2011 Feb 2. Drug Metab Dispos. 2011. PMID: 21289075 Free PMC article.
-
The cannabinoid system and its pharmacological manipulation--a review, with emphasis upon the uptake and hydrolysis of anandamide.Fundam Clin Pharmacol. 2006 Dec;20(6):549-62. doi: 10.1111/j.1472-8206.2006.00442.x. Fundam Clin Pharmacol. 2006. PMID: 17109648 Review.
-
Fatty acid amide hydrolase: an emerging therapeutic target in the endocannabinoid system.Curr Opin Chem Biol. 2003 Aug;7(4):469-75. doi: 10.1016/s1367-5931(03)00079-6. Curr Opin Chem Biol. 2003. PMID: 12941421 Review.
-
A cytochrome P450-derived epoxygenated metabolite of anandamide is a potent cannabinoid receptor 2-selective agonist.Mol Pharmacol. 2009 Apr;75(4):965-72. doi: 10.1124/mol.108.053439. Epub 2009 Jan 26. Mol Pharmacol. 2009. PMID: 19171674 Free PMC article.
-
Conformational requirements for endocannabinoid interaction with the cannabinoid receptors, the anandamide transporter and fatty acid amidohydrolase.Chem Phys Lipids. 2000 Nov;108(1-2):15-35. doi: 10.1016/s0009-3084(00)00185-7. Chem Phys Lipids. 2000. PMID: 11106780 Review.
Cited by
-
Non-opioid Analgesics and the Endocannabinoid System.Balkan Med J. 2020 Oct 23;37(6):309-315. doi: 10.4274/balkanmedj.galenos.2020.2020.6.66. Epub 2020 Jun 19. Balkan Med J. 2020. PMID: 32551466 Free PMC article.
-
Ethanolamide oxylipins of linolenic acid can negatively regulate Arabidopsis seedling development.Plant Cell. 2013 Oct;25(10):3824-40. doi: 10.1105/tpc.113.119024. Epub 2013 Oct 22. Plant Cell. 2013. PMID: 24151297 Free PMC article.
-
CYP2J2 Molecular Recognition: A New Axis for Therapeutic Design.Pharmacol Ther. 2020 Nov;215:107601. doi: 10.1016/j.pharmthera.2020.107601. Epub 2020 Jun 11. Pharmacol Ther. 2020. PMID: 32534953 Free PMC article. Review.
-
Anti-inflammatory ω-3 endocannabinoid epoxides.Proc Natl Acad Sci U S A. 2017 Jul 25;114(30):E6034-E6043. doi: 10.1073/pnas.1610325114. Epub 2017 Jul 7. Proc Natl Acad Sci U S A. 2017. PMID: 28687674 Free PMC article.
-
Alterations of Cytochrome P450s and UDP-Glucuronosyltransferases in Brain Under Diseases and Their Clinical Significances.Front Pharmacol. 2021 Apr 21;12:650027. doi: 10.3389/fphar.2021.650027. eCollection 2021. Front Pharmacol. 2021. PMID: 33967789 Free PMC article. Review.
References
-
- Alexander JP, Cravatt BF. (2006) The putative endocannabinoid transport blocker LY2183240 is a potent inhibitor of FAAH and several other brain serine hydrolases. J Am Chem Soc 128:9699–9704 - PubMed
-
- Antoun J, Amet Y, Simon B, Dréano Y, Corlu A, Corcos L, Salaun JP, Plée-Gautier E. (2006) CYP4A11 is repressed by retinoic acid in human liver cells. FEBS Lett 580:3361–3367 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

