Disruption of BCAA metabolism in mice impairs exercise metabolism and endurance
- PMID: 20133434
- PMCID: PMC2853195
- DOI: 10.1152/japplphysiol.01248.2009
Disruption of BCAA metabolism in mice impairs exercise metabolism and endurance
Abstract
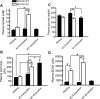
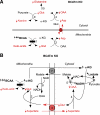
Exercise enhances branched-chain amino acid (BCAA) catabolism, and BCAA supplementation influences exercise metabolism. However, it remains controversial whether BCAA supplementation improves exercise endurance, and unknown whether the exercise endurance effect of BCAA supplementation requires catabolism of these amino acids. Therefore, we examined exercise capacity and intermediary metabolism in skeletal muscle of knockout (KO) mice of mitochondrial branched-chain aminotransferase (BCATm), which catalyzes the first step of BCAA catabolism. We found that BCATm KO mice were exercise intolerant with markedly decreased endurance to exhaustion. Their plasma lactate and lactate-to-pyruvate ratio in skeletal muscle during exercise and lactate release from hindlimb perfused with high concentrations of insulin and glucose were significantly higher in KO than wild-type (WT) mice. Plasma and muscle ammonia concentrations were also markedly higher in KO than WT mice during a brief bout of exercise. BCATm KO mice exhibited 43-79% declines in the muscle concentration of alanine, glutamine, aspartate, and glutamate at rest and during exercise. In response to exercise, the increments in muscle malate and alpha-ketoglutarate were greater in KO than WT mice. While muscle ATP concentration tended to be lower, muscle IMP concentration was sevenfold higher in KO compared with WT mice after a brief bout of exercise, suggesting elevated ammonia in KO is derived from the purine nucleotide cycle. These data suggest that disruption of BCAA transamination causes impaired malate/aspartate shuttle, thereby resulting in decreased alanine and glutamine formation, as well as increases in lactate-to-pyruvate ratio and ammonia in skeletal muscle. Thus BCAA metabolism may regulate exercise capacity in mice.
Figures





Similar articles
-
Stimulation of muscle ammonia production during exercise following branched-chain amino acid supplementation in humans.J Physiol. 1996 Jun 15;493 ( Pt 3)(Pt 3):909-22. doi: 10.1113/jphysiol.1996.sp021433. J Physiol. 1996. PMID: 8799910 Free PMC article. Clinical Trial.
-
Effect of branched-chain amino acids (BCAA), glucose, and glucose plus BCAA on endurance performance in rats.Med Sci Sports Exerc. 1999 Apr;31(4):583-7. doi: 10.1097/00005768-199904000-00015. Med Sci Sports Exerc. 1999. PMID: 10211856
-
Liver BCATm transgenic mouse model reveals the important role of the liver in maintaining BCAA homeostasis.J Nutr Biochem. 2017 Feb;40:132-140. doi: 10.1016/j.jnutbio.2016.10.014. Epub 2016 Nov 2. J Nutr Biochem. 2017. PMID: 27886623 Free PMC article.
-
Metabolism of branched-chain amino acids and ammonia during exercise: clues from McArdle's disease.Int J Sports Med. 1990 May;11 Suppl 2:S101-13. doi: 10.1055/s-2007-1024861. Int J Sports Med. 1990. PMID: 2193889 Review.
-
Branched-chain amino acids and ammonia metabolism in liver disease: therapeutic implications.Nutrition. 2013 Oct;29(10):1186-91. doi: 10.1016/j.nut.2013.01.022. Epub 2013 Jun 4. Nutrition. 2013. PMID: 23756281 Review.
Cited by
-
Metabolomic Analysis of the Skeletal Muscle of Mice Overexpressing PGC-1α.PLoS One. 2015 Jun 26;10(6):e0129084. doi: 10.1371/journal.pone.0129084. eCollection 2015. PLoS One. 2015. PMID: 26114427 Free PMC article.
-
Molecular characterization of skeletal muscle atrophy in the R6/2 mouse model of Huntington's disease.Am J Physiol Endocrinol Metab. 2011 Jul;301(1):E49-61. doi: 10.1152/ajpendo.00630.2010. Epub 2011 Apr 19. Am J Physiol Endocrinol Metab. 2011. PMID: 21505144 Free PMC article.
-
Branched-Chain Amino Acid Levels Are Related with Surrogates of Disturbed Lipid Metabolism among Older Men.Front Med (Lausanne). 2016 Nov 25;3:57. doi: 10.3389/fmed.2016.00057. eCollection 2016. Front Med (Lausanne). 2016. PMID: 27933294 Free PMC article.
-
Acute changes in blood metabolites and amino acid profile post-exercise in Foxhound dogs fed a high endurance formula.J Nutr Sci. 2014 Sep 30;3:e33. doi: 10.1017/jns.2014.46. eCollection 2014. J Nutr Sci. 2014. PMID: 26101602 Free PMC article.
-
Hypertrophy-Promoting Effects of Leucine Supplementation and Moderate Intensity Aerobic Exercise in Pre-Senescent Mice.Nutrients. 2016 May 2;8(5):246. doi: 10.3390/nu8050246. Nutrients. 2016. PMID: 27144582 Free PMC article.
References
-
- Aragon JJ, Lowenstein JM. The purine-nucleotide cycle. Comparison of the levels of citric acid cycle intermediates with the operation of the purine nucleotide cycle in rat skeletal muscle during exercise and recovery from exercise. Eur J Biochem 110: 371–377, 1980 - PubMed
-
- Babij P, Matthews SM, Rennie MJ. Changes in blood ammonia, lactate and amino acids in relation to workload during bicycle ergometer exercise in man. Eur J Appl Physiol Occup Physiol 50: 405–411, 1983 - PubMed
-
- Beckett PR. Spectrophotometric assay for measuring branched-chain amino acids. In: Methods in Enzymology Branched-chain amino acids, Part B, edited by Harris RA, Sokatch JR. New York: Academic, 2000, p. 40–47 - PubMed
-
- Block KP, Richmond WB, Mehard WB, Buse MG. Glucocorticoid-mediated activation of muscle branched-chain α-keto acid dehydrogenase in vivo. Am J Physiol Endocrinol Metab 252: E396–E407, 1987 - PubMed
-
- Blomstrand E, Ek S, Newsholme EA. Influence of ingesting a solution of branched-chain amino acids on plasma and muscle concentrations of amino acids during prolonged submaximal exercise. Nutrition 12: 485–490, 1996 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

