A phase III, randomized, double-blind, placebo-controlled study of tenecteplase for improvement of hemodialysis catheter function: TROPICS 3
- PMID: 20133491
- PMCID: PMC2849682
- DOI: 10.2215/CJN.06520909
A phase III, randomized, double-blind, placebo-controlled study of tenecteplase for improvement of hemodialysis catheter function: TROPICS 3
Abstract
Background and objectives: Despite widespread use of tunneled hemodialysis (HD) catheters, their utility is limited by the development of thrombotic complications. To address this problem, this study investigated whether the thrombolytic agent tenecteplase can restore blood flow rates (BFRs) in dysfunctional HD catheters.
Design, setting, participants, & measurements: In this randomized, double-blind study, patients with dysfunctional tunneled HD catheters, defined as a BFR <300 ml/min at -250 mmHg pressure in the arterial line, received 1-hour intracatheter dwell with tenecteplase (2 mg) or placebo. The primary endpoint was the percentage of patients with BFR > or =300 ml/min and an increase of > or =25 ml/min above baseline 30 minutes before and at the end of HD. Safety endpoints included the incidence of hemorrhagic, thrombotic, and infectious complications.
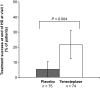
Results: Eligible patients (n = 149) were treated with tenecteplase (n = 74) or placebo (n = 75). Mean baseline BFR was similar for the tenecteplase and placebo groups at 151 and 137 ml/min, respectively. After a 1-hour dwell, 22% of patients in the tenecteplase group had functional catheters compared with 5% among placebo controls (P = 0.004). At the end of dialysis, mean change in BFR was 47 ml/min in the tenecteplase group versus 12 ml/min in the placebo group (P = 0.008). Four catheter-related bloodstream infections (one tenecteplase, three placebo) and one thrombosis (tenecteplase) were observed. There were no reports of intracranial hemorrhage, major bleeding, embolic events, or catheter-related complications.
Conclusions: Tenecteplase improved HD catheter function and had a favorable safety profile compared with placebo.
Trial registration: ClinicalTrials.gov NCT00396032.
Figures
References
-
- National Kidney Foundation: Clinical practice guidelines for vascular access. Am J Kidney Dis 48[ Suppl 1]: S248–S273, 2006 - PubMed
-
- Feldman HI, Kobrin S, Wasserstein A: Hemodialysis vascular access morbidity. J Am Soc Nephrol 7: 523–535, 1996 - PubMed
-
- Nassar GM, Ayus JC: Infectious complications of the hemodialysis access. Kidney Int 60: 1–13, 2001 - PubMed
-
- U.S. Renal Data System: USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2008
-
- Pisoni RL, Young EW, Dykstra DM, Greenwood RN, Hecking E, Gillespie B, Wolfe RA, Goodkin DA, Held PJ: Vascular access use in Europe and the United States: Results from the DOPPS. Kidney Int 1: 305–316, 2002 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical