Cell density and mobility protect swarming bacteria against antibiotics
- PMID: 20133590
- PMCID: PMC2840483
- DOI: 10.1073/pnas.0910934107
Cell density and mobility protect swarming bacteria against antibiotics
Abstract
Swarming bacteria move in multicellular groups and exhibit adaptive resistance to multiple antibiotics. Analysis of this phenomenon has revealed the protective power of high cell densities to withstand exposure to otherwise lethal antibiotic concentrations. We find that high densities promote bacterial survival, even in a nonswarming state, but that the ability to move, as well as the speed of movement, confers an added advantage, making swarming an effective strategy for prevailing against antimicrobials. We find no evidence of induced resistance pathways or quorum-sensing mechanisms controlling this group resistance, which occurs at a cost to cells directly exposed to the antibiotic. This work has relevance to the adaptive antibiotic resistance of bacterial biofilms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

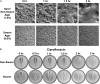

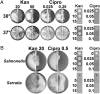

References
-
- Harshey RM. Bacterial motility on a surface: Many ways to a common goal. Annu Rev Microbiol. 2003;57:249–273. - PubMed
-
- McCarter LL. Dual flagellar systems enable motility under different circumstances. J Mol Microbiol Biotechnol. 2004;7:18–29. - PubMed
-
- Rather PN. Swarmer cell differentiation in Proteus mirabilis . Environ Microbiol. 2005;7:1065–1073. - PubMed
-
- Verstraeten N, et al. Living on a surface: Swarming and biofilm formation. Trends Microbiol. 2008;16:496–506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

