Engineered vascularized bone grafts
- PMID: 20133604
- PMCID: PMC2840421
- DOI: 10.1073/pnas.0905445107
Engineered vascularized bone grafts
Erratum in
-
Correction for Tsigkou et al., Engineered vascularized bone grafts.Proc Natl Acad Sci U S A. 2018 Sep 11;115(37):E8811. doi: 10.1073/pnas.1813106115. Epub 2018 Sep 4. Proc Natl Acad Sci U S A. 2018. PMID: 30181266 Free PMC article. No abstract available.
Abstract
Clinical protocols utilize bone marrow to seed synthetic and decellularized allogeneic bone grafts for enhancement of scaffold remodeling and fusion. Marrow-derived cytokines induce host neovascularization at the graft surface, but hypoxic conditions cause cell death at the core. Addition of cellular components that generate an extensive primitive plexus-like vascular network that would perfuse the entire scaffold upon anastomosis could potentially yield significantly higher-quality grafts. We used a mouse model to develop a two-stage protocol for generating vascularized bone grafts using mesenchymal stem cells (hMSCs) from human bone marrow and umbilical cord-derived endothelial cells. The endothelial cells formed tube-like structures and subsequently networks throughout the bone scaffold 4-7 days after implantation. hMSCs were essential for stable vasculature both in vitro and in vivo; however, contrary to expectations, vasculature derived from hMSCs briefly cultured in medium designed to maintain a proliferative, nondifferentiated state was more extensive and stable than that with hMSCs with a TGF-beta-induced smooth muscle cell phenotype. Anastomosis occurred by day 11, with most hMSCs associating closely with the network. Although initially immature and highly permeable, at 4 weeks the network was mature. Initiation of scaffold mineralization had also occurred by this period. Some human-derived vessels were still present at 5 months, but the majority of the graft vasculature had been functionally remodeled with host cells. In conclusion, clinically relevant progenitor sources for pericytes and endothelial cells can serve to generate highly functional microvascular networks for tissue engineered bone grafts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


 ;
;  ,
,  . ∗P < 0.05.)
. ∗P < 0.05.)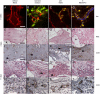
 ;
;  .)
.)
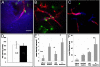
 .)
.)
 ;
;  .)
.)Similar articles
-
Vascularization of Natural and Synthetic Bone Scaffolds.Cell Transplant. 2018 Aug;27(8):1269-1280. doi: 10.1177/0963689718782452. Epub 2018 Jul 16. Cell Transplant. 2018. PMID: 30008231 Free PMC article.
-
Contrasting effects of vasculogenic induction upon biaxial bioreactor stimulation of mesenchymal stem cells and endothelial progenitor cells cocultures in three-dimensional scaffolds under in vitro and in vivo paradigms for vascularized bone tissue engineering.Tissue Eng Part A. 2013 Apr;19(7-8):893-904. doi: 10.1089/ten.TEA.2012.0187. Epub 2012 Dec 16. Tissue Eng Part A. 2013. PMID: 23102089
-
Engineering vascularized bone grafts by integrating a biomimetic periosteum and β-TCP scaffold.ACS Appl Mater Interfaces. 2014 Jun 25;6(12):9622-33. doi: 10.1021/am502056q. Epub 2014 Jun 6. ACS Appl Mater Interfaces. 2014. PMID: 24858072 Free PMC article.
-
Engineering Pre-vascularized Scaffolds for Bone Regeneration.Adv Exp Med Biol. 2015;881:79-94. doi: 10.1007/978-3-319-22345-2_5. Adv Exp Med Biol. 2015. PMID: 26545745 Review.
-
Review of vascularised bone tissue-engineering strategies with a focus on co-culture systems.J Tissue Eng Regen Med. 2015 Feb;9(2):85-105. doi: 10.1002/term.1617. Epub 2012 Nov 19. J Tissue Eng Regen Med. 2015. PMID: 23166000 Review.
Cited by
-
Analyzing Structure and Function of Vascularization in Engineered Bone Tissue by Video-Rate Intravital Microscopy and 3D Image Processing.Tissue Eng Part C Methods. 2015 Oct;21(10):1025-31. doi: 10.1089/ten.TEC.2015.0091. Epub 2015 Jul 24. Tissue Eng Part C Methods. 2015. PMID: 25962617 Free PMC article.
-
The Use of Adipose Tissue-Derived Progenitors in Bone Tissue Engineering - a Review.Transfus Med Hemother. 2016 Sep;43(5):336-343. doi: 10.1159/000447494. Epub 2016 Sep 15. Transfus Med Hemother. 2016. PMID: 27781021 Free PMC article. Review.
-
Biologically Inspired Smart Release System Based on 3D Bioprinted Perfused Scaffold for Vascularized Tissue Regeneration.Adv Sci (Weinh). 2016 Apr 15;3(8):1600058. doi: 10.1002/advs.201600058. eCollection 2016 Aug. Adv Sci (Weinh). 2016. PMID: 27818910 Free PMC article.
-
Repair of bone defects in rat radii with a composite of allogeneic adipose-derived stem cells and heterogeneous deproteinized bone.Stem Cell Res Ther. 2018 Mar 27;9(1):79. doi: 10.1186/s13287-018-0817-1. Stem Cell Res Ther. 2018. PMID: 29587852 Free PMC article.
-
Oxygen Tension-Controlled Matrices with Osteogenic and Vasculogenic Cells for Vascularized Bone Regeneration In Vivo.Tissue Eng Part A. 2016 Apr;22(7-8):610-20. doi: 10.1089/ten.TEA.2015.0310. Epub 2016 Mar 22. Tissue Eng Part A. 2016. PMID: 26914219 Free PMC article.
References
-
- Muschler GF, Nakamoto C, Griffith LG. Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am. 2004;86-A(7):1541–1558. - PubMed
-
- Folkman J, Haudenschild C. Angiogenesis in vitro. Nature. 1980;288(5791):551–556. - PubMed
-
- Sato N, et al. Development of capillary networks from rat microvascular fragments in vitro: The role of myofibroblastic cells. Microvasc Res. 1987;33(2):194–210. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

