A broad-spectrum antiviral targeting entry of enveloped viruses
- PMID: 20133606
- PMCID: PMC2840368
- DOI: 10.1073/pnas.0909587107
A broad-spectrum antiviral targeting entry of enveloped viruses
Abstract
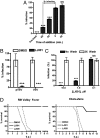
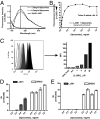
We describe an antiviral small molecule, LJ001, effective against numerous enveloped viruses including Influenza A, filoviruses, poxviruses, arenaviruses, bunyaviruses, paramyxoviruses, flaviviruses, and HIV-1. In sharp contrast, the compound had no effect on the infection of nonenveloped viruses. In vitro and in vivo assays showed no overt toxicity. LJ001 specifically intercalated into viral membranes, irreversibly inactivated virions while leaving functionally intact envelope proteins, and inhibited viral entry at a step after virus binding but before virus-cell fusion. LJ001 pretreatment also prevented virus-induced mortality from Ebola and Rift Valley fever viruses. Structure-activity relationship analyses of LJ001, a rhodanine derivative, implicated both the polar and nonpolar ends of LJ001 in its antiviral activity. LJ001 specifically inhibited virus-cell but not cell-cell fusion, and further studies with lipid biosynthesis inhibitors indicated that LJ001 exploits the therapeutic window that exists between static viral membranes and biogenic cellular membranes with reparative capacity. In sum, our data reveal a class of broad-spectrum antivirals effective against enveloped viruses that target the viral lipid membrane and compromises its ability to mediate virus-cell fusion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Viral membranes: an emerging antiviral target for enveloped viruses?Expert Rev Anti Infect Ther. 2010 Jun;8(6):635-8. doi: 10.1586/eri.10.51. Expert Rev Anti Infect Ther. 2010. PMID: 20521891
References
-
- Tam RC, Lau JY, Hong Z. Mechanisms of action of ribavirin in antiviral therapies. Antivir Chem Chemother. 2001;12:261–272. - PubMed
-
- Bekisz J, Schmeisser H, Hernandez J, Goldman ND, Zoon KC. Human interferons alpha, beta and omega. Growth Factors. 2004;22:243–251. - PubMed
-
- de Veer MJ, et al. Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol. 2001;69:912–920. - PubMed
-
- Sen GC. Viruses and interferons. Annu Rev Microbiol. 2001;55:255–281. - PubMed
-
- Hong Z, Cameron CE. Pleiotropic mechanisms of ribavirin antiviral activities. Prog Drug Res. 2002;59:41–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 AI082100/AI/NIAID NIH HHS/United States
- AI065359/AI/NIAID NIH HHS/United States
- AI082100/AI/NIAID NIH HHS/United States
- AI069317/AI/NIAID NIH HHS/United States
- T32 AI007323/AI/NIAID NIH HHS/United States
- AR053463/AR/NIAMS NIH HHS/United States
- AI070495/AI/NIAID NIH HHS/United States
- P30 AI028697/AI/NIAID NIH HHS/United States
- T32 AR053463/AR/NIAMS NIH HHS/United States
- U01 AI070495/AI/NIAID NIH HHS/United States
- R01 AI059371/AI/NIAID NIH HHS/United States
- R01 AI069317/AI/NIAID NIH HHS/United States
- AI07323/AI/NIAID NIH HHS/United States
- R21 AI084090/AI/NIAID NIH HHS/United States
- AI028697/AI/NIAID NIH HHS/United States
- U54 AI065359/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

