STAT5-glucocorticoid receptor interaction and MTF-1 regulate the expression of ZnT2 (Slc30a2) in pancreatic acinar cells
- PMID: 20133611
- PMCID: PMC2840329
- DOI: 10.1073/pnas.0914941107
STAT5-glucocorticoid receptor interaction and MTF-1 regulate the expression of ZnT2 (Slc30a2) in pancreatic acinar cells
Abstract
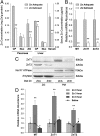
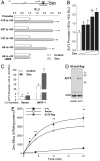
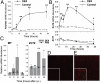
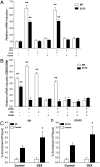
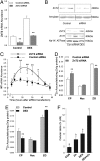
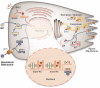
The exocrine pancreas plays an important role in endogenous zinc loss by regulating excretion into the intestinal tract and hence influences the dietary zinc requirement. The present experiments show that the zinc transporter ZnT2 (Slc30a2) is localized to the zymogen granules and that dietary zinc restriction in mice decreased the zinc concentration of zymogen granules and ZnT2 expression. Excess zinc given orally increased ZnT2 expression and was associated with increased pancreatic zinc accumulation. Rat AR42J acinar cells when induced into a secretory phenotype, using the glucocorticoid analog dexamethasone (DEX), exhibited increased ZnT2 expression and labile zinc as measured with a fluorophore. DEX administrated to mice also induced ZnT2 expression that accompanied a reduction of the pancreatic zinc content. ZnT2 promoter analyses identified elements required for responsiveness to zinc and DEX. Zinc regulation was traced to a MRE located downstream from the ZnT2 transcription start site. Responsiveness to DEX is produced by two upstream STAT5 binding sites that require the glucocorticoid receptor for activation. ZnT2 knockdown in the AR42J cells using siRNA resulted in increased cytoplasmic zinc and decreased zymogen granule zinc that further demonstrated that ZnT2 may mediate the sequestration of zinc into zymogen granules. We conclude, based upon experiments with intact mice and pancreatic acinar cells in culture, that ZnT2 participates in zinc transport into pancreatic zymogen granules through a glucocorticoid pathway requiring glucocorticoid receptor and STAT5, and zinc-regulated signaling pathways requiring MTF-1. The ZnT2 transporter appears to function in a physiologically responsive manner involving entero-pancreatic zinc trafficking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- King J, Cousins RJ. Zinc. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Modern Nutrition in Health and Disease. 10th Ed. Baltimore: Lippincott Williams and Wilkins; 2005. pp. 271–285.
-
- Lichten LA, Cousins RJ. Mammalian zinc transporters: Nutritional and physiologic regulation. Annu Rev Nutr. 2009;29:153–176. - PubMed
-
- Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem. 2006;281(34):24085–24089. - PubMed
-
- Gorelick F, Jamieson FS. Structure–Function Relations in the Pancreatic Acinar Cell. In: Johnson LR, Barrett KE, Ghishan FK, Merchant JL, Said HM, Wood JD, editors. Physiology of the Gastrointestinal Tract. 4th Ed. Vol 2. Burlington, MA: Elsevier Academic; 2006. pp. 1313–1315.
-
- Matsuno S, Miyashita E, Ejiri T, Sato T. Zinc and magnesium output in pancreatic juice after pancreaticoduodenectomy. Tohoku J Exp Med. 1982;136(1):11–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

