Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland
- PMID: 20133621
- PMCID: PMC2840294
- DOI: 10.1073/pnas.0915148107
Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland
Abstract
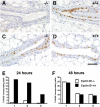
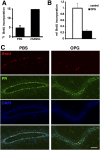
The mouse mammary gland develops postnatally under the control of female reproductive hormones. Estrogens and progesterone trigger morphogenesis by poorly understood mechanisms acting on a subset of mammary epithelial cells (MECs) that express their cognate receptors, estrogen receptor alpha (ERalpha) and progesterone receptor (PR). Here, we show that in the adult female, progesterone drives proliferation of MECs in two waves. The first, small wave, encompasses PR(+) cells and requires cyclin D1, the second, large wave, comprises mostly PR(-) cells and relies on the tumor necrosis factor (TNF) family member, receptor activator of NF-kappaB-ligand (RANKL). RANKL elicits proliferation by a paracrine mechanism. Ablation of RANKL in the mammary epithelium blocks progesterone-induced morphogenesis, and ectopic expression of RANKL in MECs completely rescues the PR(-/-) phenotype. Systemic administration of RANKL triggers proliferation in the absence of PR signaling, and injection of a RANK signaling inhibitor interferes with progesterone-induced proliferation. Thus, progesterone elicits proliferation by a cell-intrinsic and a, more important, paracrine mechanism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Targeting RANKL to a specific subset of murine mammary epithelial cells induces ordered branching morphogenesis and alveologenesis in the absence of progesterone receptor expression.FASEB J. 2010 Nov;24(11):4408-19. doi: 10.1096/fj.10-157982. Epub 2010 Jul 6. FASEB J. 2010. PMID: 20605949 Free PMC article.
-
Progesterone receptor and Stat5 signaling cross talk through RANKL in mammary epithelial cells.Mol Endocrinol. 2013 Nov;27(11):1808-24. doi: 10.1210/me.2013-1077. Epub 2013 Sep 6. Mol Endocrinol. 2013. PMID: 24014651 Free PMC article.
-
Progestin-regulated luminal cell and myoepithelial cell-specific responses in mammary organoid culture.Endocrinology. 2008 May;149(5):2098-107. doi: 10.1210/en.2007-1398. Epub 2008 Jan 24. Endocrinology. 2008. PMID: 18218689 Free PMC article.
-
The biology of progesterone receptor in the normal mammary gland and in breast cancer.Mol Cell Endocrinol. 2012 Jun 24;357(1-2):4-17. doi: 10.1016/j.mce.2011.10.030. Epub 2011 Dec 13. Mol Cell Endocrinol. 2012. PMID: 22193050 Free PMC article. Review.
-
Endocrine hormones and local signals during the development of the mouse mammary gland.Wiley Interdiscip Rev Dev Biol. 2015 May-Jun;4(3):181-95. doi: 10.1002/wdev.172. Epub 2015 Jan 23. Wiley Interdiscip Rev Dev Biol. 2015. PMID: 25645332 Review.
Cited by
-
The association between RANKL and Osteoprotegerin gene polymorphisms with breast cancer.Mol Cell Biochem. 2015 May;403(1-2):219-29. doi: 10.1007/s11010-015-2352-z. Epub 2015 Feb 28. Mol Cell Biochem. 2015. PMID: 25724681
-
Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer.Endocr Rev. 2013 Feb;34(1):130-62. doi: 10.1210/er.2012-1043. Epub 2013 Jan 9. Endocr Rev. 2013. PMID: 23303565 Free PMC article. Review.
-
Megestrol Acetate Increases the Proliferation, Migration, and Adipogenic Differentiation of Adipose-Derived Stem Cells via Glucocorticoid Receptor.Stem Cells Transl Med. 2015 Jul;4(7):789-99. doi: 10.5966/sctm.2015-0009. Epub 2015 May 13. Stem Cells Transl Med. 2015. PMID: 25972147 Free PMC article.
-
TNFAIP3 Reduction-of-Function Drives Female Infertility and CNS Inflammation.Front Immunol. 2022 Apr 8;13:811525. doi: 10.3389/fimmu.2022.811525. eCollection 2022. Front Immunol. 2022. PMID: 35464428 Free PMC article.
-
Progesterone and Breast Cancer.Endocr Rev. 2020 Apr 1;41(2):320-44. doi: 10.1210/endrev/bnz001. Endocr Rev. 2020. PMID: 31512725 Free PMC article. Review.
References
-
- Colditz GA, Rosner BA, Chen WY, Holmes MD, Hankinson SE. Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst. 2004;96:218–228. - PubMed
-
- Dupont S, et al. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. - PubMed
-
- Lydon JP, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. - PubMed
-
- Ormandy CJ, et al. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997;11:167–178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

