Persistent hepatitis C virus infection in microscale primary human hepatocyte cultures
- PMID: 20133632
- PMCID: PMC2840339
- DOI: 10.1073/pnas.0915130107
Persistent hepatitis C virus infection in microscale primary human hepatocyte cultures
Abstract
Hepatitis C virus (HCV) remains a major public health problem, affecting approximately 130 million people worldwide. HCV infection can lead to cirrhosis, hepatocellular carcinoma, and end-stage liver disease, as well as extrahepatic complications such as cryoglobulinemia and lymphoma. Preventative and therapeutic options are severely limited; there is no HCV vaccine available, and nonspecific, IFN-based treatments are frequently ineffective. Development of targeted antivirals has been hampered by the lack of robust HCV cell culture systems that reliably predict human responses. Here, we show the entire HCV life cycle recapitulated in micropatterned cocultures (MPCCs) of primary human hepatocytes and supportive stroma in a multiwell format. MPCCs form polarized cell layers expressing all known HCV entry factors and sustain viral replication for several weeks. When coupled with highly sensitive fluorescence- and luminescence-based reporter systems, MPCCs have potential as a high-throughput platform for simultaneous assessment of in vitro efficacy and toxicity profiles of anti-HCV therapeutics.
Conflict of interest statement
The authors declare no conflict of interest. Materials used as controls in this study, the HCVcc cell culture virus system and Huh-7.5 hepatoma cells and reporter derivatives, were created at Washington University or Rockefeller University. These were then licensed to a commercial entity, Apath LLC, in which C.M.R. holds equity.
Figures
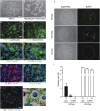
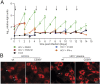
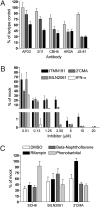
Comment in
-
Getting closer to the patient: upgrade of hepatitis C virus infection in primary human hepatocytes.J Hepatol. 2010 Aug;53(2):388-9. doi: 10.1016/j.jhep.2010.04.004. Epub 2010 Apr 29. J Hepatol. 2010. PMID: 20546960 No abstract available.
References
-
- Durantel D, Zoulim F. Going towards more relevant cell culture models to study the in vitro replication of serum-derived hepatitis C virus and virus/host cell interactions? J Hepatol. 2007;46:1–5. - PubMed
-
- Hsu IC, et al. p53 gene mutation and integrated hepatitis B viral DNA sequences in human liver cancer cell lines. Carcinogenesis. 1993;14:987–992. - PubMed
-
- Nagao K, et al. Expression of hTERT mRNA in a mortal liver cell line during S phase without detectable telomerase activity. Int J Mol Med. 2005;15:683–688. - PubMed
-
- Yokoo H, et al. Proteomic signature corresponding to alpha fetoprotein expression in liver cancer cells. Hepatology. 2004;40:609–617. - PubMed
-
- Fournier C, et al. In vitro infection of adult normal human hepatocytes in primary culture by hepatitis C virus. J Gen Virol. 1998;79:2367–2374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

