Molecular defects of the glycine 41 variants of alanine glyoxylate aminotransferase associated with primary hyperoxaluria type I
- PMID: 20133649
- PMCID: PMC2840350
- DOI: 10.1073/pnas.0908565107
Molecular defects of the glycine 41 variants of alanine glyoxylate aminotransferase associated with primary hyperoxaluria type I
Abstract
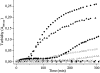
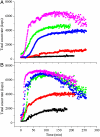
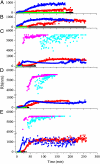
G41 is an interfacial residue located within the alpha-helix 34-42 of alanine:glyoxylate aminotransferase (AGT). Its mutations on the major (AGT-Ma) or the minor (AGT-Mi) allele give rise to the variants G41R-Ma, G41R-Mi, and G41V-Ma causing hyperoxaluria type 1. Impairment of dimerization in these variants has been suggested to be responsible for immunoreactivity deficiency, intraperoxisomal aggregation, and sensitivity to proteasomal degradation. However, no experimental evidence supports this view. Here we report that G41 mutations, besides increasing the dimer-monomer equilibrium dissociation constant, affect the protein conformation and stability, and perturb its active site. As compared to AGT-Ma or AGT-Mi, G41 variants display different near-UV CD and intrinsic emission fluorescence spectra, larger exposure of hydrophobic surfaces, sensitivity to Met53-Tyr54 peptide bond cleavage by proteinase K, decreased thermostability, reduced coenzyme binding affinity, and catalytic efficiency. Additionally, unlike AGT-Ma and AGT-Mi, G41 variants under physiological conditions form insoluble inactive high-order aggregates (approximately 5,000 nm) through intermolecular electrostatic interactions. A comparative molecular dynamics study of the putative structures of AGT-Mi and G41R-Mi predicts that G41 --> R mutation causes a partial unwinding of the 34-42 alpha-helix and a displacement of the first 44 N-terminal residues including the active site loop 24-32. These simulations help us to envisage the possible structural basis of AGT dysfunction associated with G41 mutations. The detailed insight into how G41 mutations act on the structure-function of AGT may contribute to achieve the ultimate goal of correcting the effects of these mutations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Zhang X, et al. Crystal structure of alanine:glyoxylate aminotransferase and the relationship between genotype and enzymatic phenotype in primary hyperoxaluria type 1. J Mol Biol. 2003;331(3):643–652. - PubMed
-
- Motley A, et al. Mammalian alanine/glyoxylate aminotransferase 1 is imported into peroxisomes via the PTS1 translocation pathway. Increased degeneracy and context specificity of the mammalian PTS1 motif and implications for the peroxisome-to-mitochondrion mistargeting of AGT in primary hyperoxaluria type 1. J Cell Biol. 1995;131(1):95–109. - PMC - PubMed
-
- Danpure CJ. Molecular etiology of primary hyperoxaluria type 1: New directions for treatment. Am J Nephrol. 2005;25(3):303–310. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

