Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis
- PMID: 20133650
- PMCID: PMC2840492
- DOI: 10.1073/pnas.0914798107
Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis
Abstract
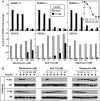
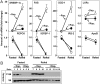
The livers of insulin-resistant, diabetic mice manifest selective insulin resistance, suggesting a bifurcation in the insulin signaling pathway: Insulin loses its ability to block glucose production (i.e., it fails to suppress PEPCK and other genes of gluconeogenesis), yet it retains its ability to stimulate fatty acid synthesis (i.e., continued enhancement of genes of lipogenesis). Enhanced lipogenesis is accompanied by an insulin-stimulated increase in the mRNA encoding SREBP-1c, a transcription factor that activates the entire lipogenic program. Here, we report a branch point in the insulin signaling pathway that may account for selective insulin resistance. Exposure of rat hepatocytes to insulin produced a 25-fold increase in SREBP-1c mRNA and a 95% decrease in PEPCK mRNA. Insulin-mediated changes in both mRNAs were blocked by inhibitors of PI3K and Akt, indicating that these kinases are required for both pathways. In contrast, subnanomolar concentrations of rapamycin, an inhibitor of the mTORC1 kinase, blocked insulin induction of SREBP-1c, but had no effect on insulin suppression of PEPCK. We observed a similar selective effect of rapamycin in livers of rats and mice that experienced an insulin surge in response to a fasting-refeeding protocol. A specific inhibitor of S6 kinase, a downstream target of mTORC1, did not block insulin induction of SREBP-1c, suggesting a downstream pathway distinct from S6 kinase. These results establish mTORC1 as an essential component in the insulin-regulated pathway for hepatic lipogenesis but not gluconeogenesis, and may help to resolve the paradox of selective insulin resistance in livers of diabetic rodents.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
mTORC1 activates SREBP-1c and uncouples lipogenesis from gluconeogenesis.Proc Natl Acad Sci U S A. 2010 Feb 23;107(8):3281-2. doi: 10.1073/pnas.1000323107. Epub 2010 Feb 18. Proc Natl Acad Sci U S A. 2010. PMID: 20167806 Free PMC article. No abstract available.
References
-
- Reaven GM. Why syndrome X? From Harold Himsworth to the insulin resistance syndrome. Cell Metab. 2005;1:9–14. - PubMed
-
- Brown MS, Goldstein JL. Selective vs total insulin resistance: A pathogenic paradox. Cell Metab. 2008;7:95–96. - PubMed
-
- Shimomura I, et al. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000;6:77–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

