Structure of Vibrio cholerae ToxT reveals a mechanism for fatty acid regulation of virulence genes
- PMID: 20133655
- PMCID: PMC2840316
- DOI: 10.1073/pnas.0915021107
Structure of Vibrio cholerae ToxT reveals a mechanism for fatty acid regulation of virulence genes
Abstract
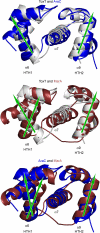
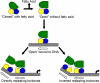
Cholera is an acute intestinal infection caused by the bacterium Vibrio cholerae. In order for V. cholerae to cause disease, it must produce two virulence factors, the toxin-coregulated pilus (TCP) and cholera toxin (CT), whose expression is controlled by a transcriptional cascade culminating with the expression of the AraC-family regulator, ToxT. We have solved the 1.9 A resolution crystal structure of ToxT, which reveals folds in the N- and C-terminal domains that share a number of features in common with AraC, MarA, and Rob as well as the unexpected presence of a buried 16-carbon fatty acid, cis-palmitoleate. The finding that cis-palmitoleic acid reduces TCP and CT expression in V. cholerae and prevents ToxT from binding to DNA in vitro provides a direct link between the host environment of V. cholerae and regulation of virulence gene expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
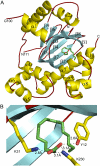


References
-
- Champion GA, Neely MN, Brennan MA, DiRita VJ. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol Microbiol. 1997;23:323–331. - PubMed
-
- Brown RC, Taylor RK. Organization of tcp, acf, and toxT genes within a ToxT-dependent operon. Mol Microbiol. 1995;16:425–439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

