Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure
- PMID: 20133658
- PMCID: PMC2823910
- DOI: 10.1073/pnas.0910174107
Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure
Abstract
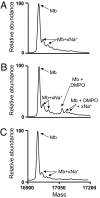
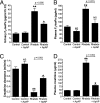
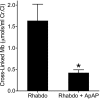
Hemoproteins, hemoglobin and myoglobin, once released from cells can cause severe oxidative damage as a consequence of heme redox cycling between ferric and ferryl states that generates radical species that induce lipid peroxidation. We demonstrate in vitro that acetaminophen inhibits hemoprotein-induced lipid peroxidation by reducing ferryl heme to its ferric state and quenching globin radicals. Severe muscle injury (rhabdomyolysis) is accompanied by the release of myoglobin that becomes deposited in the kidney, causing renal injury. We previously showed in a rat model of rhabdomyolysis that redox cycling between ferric and ferryl myoglobin yields radical species that cause severe oxidative damage to the kidney. In this model, acetaminophen at therapeutic plasma concentrations significantly decreased oxidant injury in the kidney, improved renal function, and reduced renal damage. These findings also provide a hypothesis for potential therapeutic applications for acetaminophen in diseases involving hemoprotein-mediated oxidative injury.
Conflict of interest statement
Conflict of interest statement: J.A.O., O.B., and L.J.R. have filed a patent for the use of acetaminophen in rhabdomyolysis-induced renal failure. J.A.O. is a consultant for McNeil Pharmaceuticals.
Figures



References
-
- Grisham MB. Myoglobin-catalyzed hydrogen peroxide dependent arachidonic acid peroxidation. J Free Radic Biol Med. 1985;1:227–232. - PubMed
-
- Harel S, Kanner J. The generation of ferryl or hydroxyl radicals during interaction of haemproteins with hydrogen peroxide. Free Radic Res Commun. 1988;5:21–33. - PubMed
-
- Hogg N, et al. The role of lipid hydroperoxides in the myoglobin-dependent oxidation of LDL. Arch Biochem Biophys. 1994;314:39–44. - PubMed
-
- Patel RP, Svistunenko DA, Darley-Usmar VM, Symons MC, Wilson MT. Redox cycling of human methaemoglobin by H2O2 yields persistent ferryl iron and protein based radicals. Free Radic Res. 1996;25:117–123. - PubMed
-
- Ortiz de Montellano PR, Catalano CE. Epoxidation of styrene by hemoglobin and myoglobin. Transfer of oxidizing equivalents to the protein surface. J Biol Chem. 1985;260:9265–9271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK48831/DK/NIDDK NIH HHS/United States
- GM15431/GM/NIGMS NIH HHS/United States
- R01 DK048831/DK/NIDDK NIH HHS/United States
- R03 AI060827/AI/NIAID NIH HHS/United States
- P01 GM015431/GM/NIGMS NIH HHS/United States
- R37 GM042056/GM/NIGMS NIH HHS/United States
- P50 GM015431/GM/NIGMS NIH HHS/United States
- GM42056/GM/NIGMS NIH HHS/United States
- AI060827/AI/NIAID NIH HHS/United States
- G0800971/MRC_/Medical Research Council/United Kingdom
- BBF0076631/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- R01 GM042056/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

