Analysis of a gain-of-function FGFR2 Crouzon mutation provides evidence of loss of function activity in the etiology of cleft palate
- PMID: 20133659
- PMCID: PMC2823872
- DOI: 10.1073/pnas.0913985107
Analysis of a gain-of-function FGFR2 Crouzon mutation provides evidence of loss of function activity in the etiology of cleft palate
Abstract
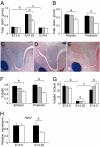
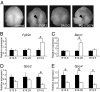
Cleft palate is a common birth defect in humans and is a common phenotype associated with syndromic mutations in fibroblast growth factor receptor 2 (Fgfr2). Cleft palate occurred in nearly all mice homozygous for the Crouzon syndrome mutation C342Y in the mesenchymal splice form of Fgfr2. Mutant embryos showed delayed palate elevation, stage-specific biphasic changes in palate mesenchymal proliferation, and reduced levels of mesenchymal glycosaminoglycans (GAGs). Reduced levels of feedback regulators of FGF signaling suggest that this gain-of-function mutation in FGFR2 ultimately resembles loss of FGF function in palate mesenchyme. Knowledge of how mesenchymal FGF signaling regulates palatal shelf development may ultimately lead to pharmacological approaches to reduce cleft palate incidence in genetically predisposed humans.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Type 1 fibroblast growth factor receptor in cranial neural crest cell-derived mesenchyme is required for palatogenesis.J Biol Chem. 2013 Jul 26;288(30):22174-83. doi: 10.1074/jbc.M113.463620. Epub 2013 Jun 10. J Biol Chem. 2013. PMID: 23754280 Free PMC article.
-
Mesenchymal fibroblast growth factor receptor signaling regulates palatal shelf elevation during secondary palate formation.Dev Dyn. 2015 Nov;244(11):1427-38. doi: 10.1002/dvdy.24319. Epub 2015 Aug 24. Dev Dyn. 2015. PMID: 26250517 Free PMC article.
-
Inactivation of Fgfr2 gene in mouse secondary palate mesenchymal cells leads to cleft palate.Reprod Toxicol. 2018 Apr;77:137-142. doi: 10.1016/j.reprotox.2018.03.004. Epub 2018 Mar 8. Reprod Toxicol. 2018. PMID: 29526646
-
Development of the lip and palate: FGF signalling.Front Oral Biol. 2012;16:71-80. doi: 10.1159/000337618. Epub 2012 Jun 25. Front Oral Biol. 2012. PMID: 22759671 Review.
-
A review of FGF signaling in palate development.Biomed Pharmacother. 2018 Jul;103:240-247. doi: 10.1016/j.biopha.2018.04.026. Epub 2018 Apr 24. Biomed Pharmacother. 2018. PMID: 29655165 Review.
Cited by
-
From shape to cells: mouse models reveal mechanisms altering palate development in Apert syndrome.Dis Model Mech. 2013 May;6(3):768-79. doi: 10.1242/dmm.010397. Epub 2013 Mar 8. Dis Model Mech. 2013. PMID: 23519026 Free PMC article.
-
The Evaluation of FGFR1, FGFR2 and FOXO1 in Orofacial Cleft Tissue.Children (Basel). 2022 Apr 6;9(4):516. doi: 10.3390/children9040516. Children (Basel). 2022. PMID: 35455561 Free PMC article.
-
SPECC1L regulates palate development downstream of IRF6.Hum Mol Genet. 2020 Mar 27;29(5):845-858. doi: 10.1093/hmg/ddaa002. Hum Mol Genet. 2020. PMID: 31943082 Free PMC article.
-
Palate morphogenesis: current understanding and future directions.Birth Defects Res C Embryo Today. 2010 Jun;90(2):133-54. doi: 10.1002/bdrc.20180. Birth Defects Res C Embryo Today. 2010. PMID: 20544696 Free PMC article. Review.
-
Revisiting the embryogenesis of lip and palate development.Oral Dis. 2022 Jul;28(5):1306-1326. doi: 10.1111/odi.14174. Epub 2022 Mar 5. Oral Dis. 2022. PMID: 35226783 Free PMC article. Review.
References
-
- Wilkie AO. Bad bones, absent smell, selfish testes: The pleiotropic consequences of human FGF receptor mutations. Cytokine Growth Factor Rev. 2005;16:187–203. - PubMed
-
- Hajihosseini MK. Fibroblast growth factor signaling in cranial suture development and pathogenesis. Front Oral Biol. 2008;12:160–177. - PubMed
-
- Marie PJ, Coffin JD, Hurley MM. FGF and FGFR signaling in chondrodysplasias and craniosynostosis. J Cell Biochem. 2005;96:888–896. - PubMed
-
- Ornitz DM, Marie PJ. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev. 2002;16:1446–1465. - PubMed
-
- McDowell LM, et al. Inhibition or activation of Apert syndrome FGFR2 (S252W) signaling by specific glycosaminoglycans. J Biol Chem. 2006;281:6924–6930. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

