Supramolecular design of self-assembling nanofibers for cartilage regeneration
- PMID: 20133666
- PMCID: PMC2840471
- DOI: 10.1073/pnas.0906501107
Supramolecular design of self-assembling nanofibers for cartilage regeneration
Abstract
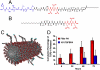
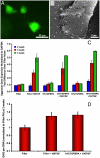
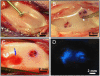
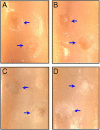
Molecular and supramolecular design of bioactive biomaterials could have a significant impact on regenerative medicine. Ideal regenerative therapies should be minimally invasive, and thus the notion of self-assembling biomaterials programmed to transform from injectable liquids to solid bioactive structures in tissue is highly attractive for clinical translation. We report here on a coassembly system of peptide amphiphile (PA) molecules designed to form nanofibers for cartilage regeneration by displaying a high density of binding epitopes to transforming growth factor beta-1 (TGFbeta-1). Growth factor release studies showed that passive release of TGFbeta-1 was slower from PA gels containing the growth factor binding sites. In vitro experiments indicate these materials support the survival and promote the chondrogenic differentiation of human mesenchymal stem cells. We also show that these materials can promote regeneration of articular cartilage in a full thickness chondral defect treated with microfracture in a rabbit model with or even without the addition of exogenous growth factor. These results demonstrate the potential of a completely synthetic bioactive biomaterial as a therapy to promote cartilage regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Buckwalter JA, Mankin HJ. Articular cartilage: Degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. - PubMed
-
- O’Driscoll SW. Current concepts review—The healing and regeneration of articular cartilage. J Bone Joint Surg Am. 1998;80:1795–1812. - PubMed
-
- Hunziker EB. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthr Cartilage. 2002;10:432–463. - PubMed
-
- Jackson DW, Simon TM, Aberman HM. Symptomatic articular cartilage degeneration: The impact in the new millennium. Clin Orthop Relat Res. 2001;391 (Suppl):S14–25. - PubMed
-
- Williams RJ, Harnly HW. Microfracture: Indications, technique, and results. Instr Course Lect. 2007;56:419–428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

