Competing feedback loops shape IL-2 signaling between helper and regulatory T lymphocytes in cellular microenvironments
- PMID: 20133667
- PMCID: PMC2840293
- DOI: 10.1073/pnas.0812851107
Competing feedback loops shape IL-2 signaling between helper and regulatory T lymphocytes in cellular microenvironments
Abstract
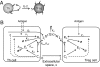
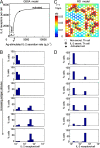
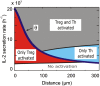
Cytokines are pleiotropic and readily diffusible messenger molecules, raising the question of how their action can be confined to specific target cells. The T cell cytokine interleukin-2 (IL-2) is essential for the homeostasis of regulatory T (Treg) cells that suppress (auto)immunity and stimulates immune responses mediated by conventional T cells. We combined mathematical modeling and experiments to dissect the dynamics of the IL-2 signaling network that links the prototypical IL-2 producers, conventional T helper (Th) cells, and Treg cells. We show how the IL-2-induced upregulation of high-affinity IL-2 receptors (IL-2R) establishes a positive feedback loop of IL-2 signaling. This feedback mediates a digital switch for the proliferation of Th cells and functions as an analog amplifier for the IL-2 uptake capacity of Treg cells. Unlike other positive feedbacks in cell signaling that augment signal propagation, the IL-2/IL-2R loop enhances the capture of the signal molecule and its degradation. Thus Treg and Th cells can compete for IL-2 and restrict its range of action through efficient cellular uptake. Depending on activation status and spatial localization of the cells, IL-2 may be consumed exclusively by Treg or Th cells, or be shared between them. In particular, a Treg cell can deprive a stimulated Th cell of its IL-2, but only when the cells are located in close proximity, within a few tens of micrometers. The present findings explain how IL-2 can play two distinct roles in immune regulation and point to a hitherto largely unexplored spatiotemporal complexity of cytokine signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Cantrell DA, Smith KA. The interleukin-2 T-cell system: A new cell growth model. Science. 1984;224:1312–1316. - PubMed
-
- Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. - PubMed
-
- Shevach EM. Regulatory T cells in autoimmunity. Annu Rev Immunol. 2000;18:423–449. - PubMed
-
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

