Proliferative lesions and metalloproteinase activity in murine lupus nephritis mediated by type I interferons and macrophages
- PMID: 20133703
- PMCID: PMC2840310
- DOI: 10.1073/pnas.0914902107
Proliferative lesions and metalloproteinase activity in murine lupus nephritis mediated by type I interferons and macrophages
Abstract
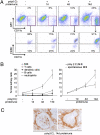
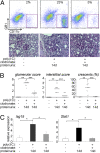
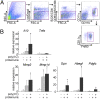
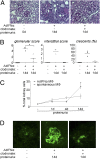
Glomerulonephritis is a major cause of morbidity in patients with systemic lupus erythematosus. Although substantial progress has been made in the identification of pathogenic triggers that result in autoantibody production, little is known about the pathogenesis of aggressive proliferative processes that lead directly to irreversible glomerular damage and compromise of renal function. In this study, we describe a model of polyinosinic: polycytidylic acid-accelerated lupus nephritis in NZB/W mice that is characterized by severe glomerular proliferative lesions with de novo crescent formation, findings that are linked with decreased survival and adverse outcomes in lupus. Proliferative glomerulonephritis was associated with infiltrating kidney macrophages and renal expression of IFN-inducible genes, matrix metalloproteinases (MMPs), and growth factors. Crescent formation and renal MMP and growth factor expression were dependent on renal macrophages that expressed Il10, MMPs, osteopontin, and growth factors, including Pdgfc and Hbegf. Infiltrating macrophages and renal MMP expression were induced by type I IFN. These findings reveal a role for type I IFNs and alternatively activated macrophages in aggressive proliferative lesions of lupus nephritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Schwartz MM, Korbet SM, Katz RS, Lewis EJ. Evidence of concurrent immuno-pathological mechanisms determining the pathology of severe lupus nephritis. Lupus. 2009;18:149–158. - PubMed
-
- Kojo S, et al. Clinical usefulness of a prognostic score in histological analysis of renal biopsy in patients with lupus nephritis. J Rheumatol. 2009;36:2218–2223. - PubMed
-
- Hill GS, et al. Predictive power of the second renal biopsy in lupus nephritis: significance of macrophages. Kidney Int. 2001;59:304–316. - PubMed
-
- Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–551. - PubMed
-
- Davidson A, Aranow C. Pathogenesis and treatment of systemic lupus erythematosus nephritis. Curr Opin Rheumatol. 2006;18:468–475. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

