PML/RARalpha fusion protein transactivates the tissue factor promoter through a GAGC-containing element without direct DNA association
- PMID: 20133705
- PMCID: PMC2840450
- DOI: 10.1073/pnas.0915006107
PML/RARalpha fusion protein transactivates the tissue factor promoter through a GAGC-containing element without direct DNA association
Abstract
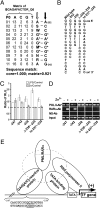
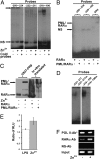
A severe coagulopathy is a life-threatening complication of acute promyelocytic leukemia (APL) and is ascribable mainly to the excessive levels of tissue factor (TF) in APL cells regulated in response to the promyelocytic leukemia/retinoic acid receptor alpha (PML/RARalpha) fusion protein. The underlying molecular mechanisms for this regulation remain ill-defined. With U937-PR9 cell lines stably expressing luciferase reporter gene under the control of different mutants of the TF promoter, both luciferase and ChIP data allowed the localization of the PML/RARalpha-responsive sequence in a previously undefined region of the TF promoter at position -230 to -242 devoid of known mammalian transcription factor binding sites. Within this sequence a GAGC motif (-235 to -238) was shown to be crucial because deletion or mutation of these nucleotides impaired both PML/RARalpha interaction and promoter transactivation. However, EMSA results showed that PML/RARalpha did not bind to DNA probes encompassing the -230 to -242 sequences, precluding a direct DNA association. Mutational experiments further suggest that the activator protein 1 (AP-1) sites of the TF promoter are dispensable for PML/RARalpha regulation. This study shows that PML/RARalpha transactivates the TF promoter through an indirect interaction with an element composed of a GAGC motif and the flanking nucleotides, independent of AP-1 binding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Chong BH, Lee SH. Management of thromboembolism in hematologic malignancies. Semin Thromb Hemost. 2007;33:435–448. - PubMed
-
- Rickles FR, et al. Bleeding and thrombosis in acute leukemia: What does the future of therapy look like? Thromb Res. 2007;120(Suppl 2):S99–S106. - PubMed
-
- Tallman MS, Kwaan HC. Intravascular clotting activation and bleeding in patients with hematologic malignancies. Rev Clin Exp Hematol. 2004;8:E1. - PubMed
-
- Kwaan HC, Wang J, Boggio LN. Abnormalities in hemostasis in acute promyelocytic leukemia. Hematol Oncol. 2002;20:33–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

