LXR modulation blocks prostaglandin E2 production and matrix degradation in cartilage and alleviates pain in a rat osteoarthritis model
- PMID: 20133709
- PMCID: PMC2840473
- DOI: 10.1073/pnas.0911377107
LXR modulation blocks prostaglandin E2 production and matrix degradation in cartilage and alleviates pain in a rat osteoarthritis model
Abstract
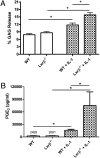
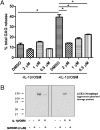
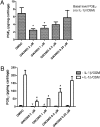
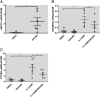
Osteoarthritis (OA), the most common arthritic condition in humans, is characterized by the progressive degeneration of articular cartilage accompanied by chronic joint pain. Inflammatory mediators, such as cytokines and prostaglandin E(2) (PGE(2)) that are elevated in OA joints, play important roles in the progression of cartilage degradation and pain-associated nociceptor sensitivity. We have found that the nuclear receptor family transcription factors Liver X Receptors (LXRalpha and -beta) are expressed in cartilage, with LXRbeta being the predominant isoform. Here we show that genetic disruption of Lxrbeta gene expression in mice results in significantly increased proteoglycan (aggrecan) degradation and PGE(2) production in articular cartilage treated with IL-1beta, indicating a protective role of LXRbeta in cartilage. Using human cartilage explants, we found that activation of LXRs by the synthetic ligand GW3965 significantly reduced cytokine-induced degradation and loss of aggrecan from the tissue. Furthermore, LXR activation dramatically inhibited cytokine-induced PGE(2) production by human osteoarthritic cartilage as well as by a synovial sarcoma cell line. These effects were achieved at least partly by repression of the expression of ADAMTS4, a physiological cartilage aggrecanase, and of cyclooxygenase-2 and microsomal prostaglandin E synthase-1, key enzymes in the PGE(2) synthesis pathway. Consistent with our in vitro observations, oral administration of GW3965 potently alleviated joint pain in a rat meniscal tear model of osteoarthritis.
Conflict of interest statement
Conflict of interest statement: All authors are Pfizer (formerly Wyeth Research) employees. The research is funded by Wyeth Research.
Figures


Similar articles
-
Effects of glucosamine and chondroitin sulfate on bovine cartilage explants under long-term culture conditions.Am J Vet Res. 2007 Jul;68(7):709-15. doi: 10.2460/ajvr.68.7.709. Am J Vet Res. 2007. PMID: 17605605
-
Activation of LXRs using the synthetic agonist GW3965 represses the production of pro-inflammatory cytokines by murine mast cells.Allergol Int. 2015 Sep;64 Suppl:S11-7. doi: 10.1016/j.alit.2015.03.001. Epub 2015 Mar 31. Allergol Int. 2015. PMID: 26344074
-
In vitro inhibition of aggrecanase activity by tetracyclines and proteoglycan loss from osteoarthritic human articular cartilage.J Orthop Res. 2010 Jun;28(6):828-33. doi: 10.1002/jor.21026. J Orthop Res. 2010. PMID: 20069635
-
Suppression of aggrecanase: a novel protective mechanism of dehydroepiandrosterone in osteoarthritis?Mol Biol Rep. 2010 Mar;37(3):1241-5. doi: 10.1007/s11033-009-9495-5. Epub 2009 Mar 10. Mol Biol Rep. 2010. PMID: 19277895 Review.
-
Aggrecanase and aggrecan degradation in osteoarthritis: a review.J Int Med Res. 2008 Nov-Dec;36(6):1149-60. doi: 10.1177/147323000803600601. J Int Med Res. 2008. PMID: 19094423 Review.
Cited by
-
Nuclear receptors regulate lipid metabolism and oxidative stress markers in chondrocytes.J Mol Med (Berl). 2017 Apr;95(4):431-444. doi: 10.1007/s00109-016-1501-5. Epub 2017 Jan 9. J Mol Med (Berl). 2017. PMID: 28070626 Free PMC article.
-
Targeting liver X receptors in cancer therapeutics.Nat Rev Cancer. 2015 Apr;15(4):216-24. doi: 10.1038/nrc3912. Epub 2015 Mar 19. Nat Rev Cancer. 2015. PMID: 25786697 Review.
-
Epigenetic Up-Regulation of ADAMTS4 in Sympathetic Ganglia is Involved in the Maintenance of Neuropathic Pain Following Nerve Injury.Neurochem Res. 2023 Aug;48(8):2350-2359. doi: 10.1007/s11064-023-03896-x. Epub 2023 Mar 22. Neurochem Res. 2023. PMID: 36947308
-
LXR Suppresses Inflammatory Gene Expression and Neutrophil Migration through cis-Repression and Cholesterol Efflux.Cell Rep. 2018 Dec 26;25(13):3774-3785.e4. doi: 10.1016/j.celrep.2018.11.100. Cell Rep. 2018. PMID: 30590048 Free PMC article.
-
Hair follicular expression and function of group X secreted phospholipase A2 in mouse skin.J Biol Chem. 2011 Apr 1;286(13):11616-31. doi: 10.1074/jbc.M110.206714. Epub 2011 Jan 25. J Biol Chem. 2011. PMID: 21266583 Free PMC article.
References
-
- Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237–246. - PubMed
-
- Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004;(427 Suppl):S27–S36. - PubMed
-
- El Mabrouk M, Sylvester J, Zafarullah M. Signaling pathways implicated in oncostatin M-induced aggrecanase-1 and matrix metalloproteinase-13 expression in human articular chondrocytes. Biochim Biophys Acta. 2007;1773:309–320. - PubMed
-
- Glasson SS, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. - PubMed
-
- Mastbergen SC, Bijlsma JW, Lafeber FP. Selective COX-2 inhibition is favorable to human early and late-stage osteoarthritic cartilage: A human in vitro study. Osteoarthritis Cartilage. 2005;13:519–526. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

