TDP-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration
- PMID: 20133711
- PMCID: PMC2840518
- DOI: 10.1073/pnas.0912417107
TDP-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration
Abstract
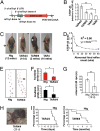
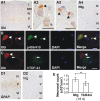
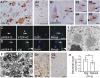
Neuronal cytoplasmic and intranuclear aggregates of RNA-binding protein TDP-43 are a hallmark feature of neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD). ALS and FTLD show a considerable clinical and pathological overlap and occur as both familial and sporadic forms. Though missense mutations in TDP-43 cause rare forms of familial ALS, it is not yet known whether this is due to loss of TDP-43 function or gain of aberrant function. Moreover, the role of wild-type (WT) TDP-43, associated with the majority of familial and sporadic ALS/FTLD patients, is also currently unknown. Generating homozygous and hemizygous WT human TDP-43 transgenic mouse lines, we show here a dose-dependent degeneration of cortical and spinal motor neurons and development of spastic quadriplegia reminiscent of ALS. A dose-dependent degeneration of nonmotor cortical and subcortical neurons characteristic of FTLD was also observed. Neurons in the affected spinal cord and brain regions showed accumulation of TDP-43 nuclear and cytoplasmic aggregates that were both ubiquitinated and phosphorylated as observed in ALS/FTLD patients. Moreover, the characteristic approximately 25-kDa C-terminal fragments (CTFs) were also recovered from nuclear fractions and correlated with disease development and progression in WT TDP-43 mice. These findings suggest that approximately 25-kDa TDP-43 CTFs are noxious to neurons by a gain of aberrant nuclear function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Distinct TDP-43 inclusion morphologies in frontotemporal lobar degeneration with and without amyotrophic lateral sclerosis.Acta Neuropathol Commun. 2017 Oct 27;5(1):76. doi: 10.1186/s40478-017-0480-2. Acta Neuropathol Commun. 2017. PMID: 29078806 Free PMC article.
-
Dysregulation of the ALS-associated gene TDP-43 leads to neuronal death and degeneration in mice.J Clin Invest. 2011 Feb;121(2):726-38. doi: 10.1172/JCI44867. Epub 2011 Jan 4. J Clin Invest. 2011. PMID: 21206091 Free PMC article.
-
The RNA-binding motif 45 (RBM45) protein accumulates in inclusion bodies in amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration with TDP-43 inclusions (FTLD-TDP) patients.Acta Neuropathol. 2012 Nov;124(5):717-32. doi: 10.1007/s00401-012-1045-x. Epub 2012 Sep 21. Acta Neuropathol. 2012. PMID: 22993125 Free PMC article.
-
[Clinical and pathological spectrum of TDP-43 associated ALS].Rinsho Shinkeigaku. 2010 Nov;50(11):940-2. doi: 10.5692/clinicalneurol.50.940. Rinsho Shinkeigaku. 2010. PMID: 21921519 Review. Japanese.
-
Possible concurrence of TDP-43, tau and other proteins in amyotrophic lateral sclerosis/frontotemporal lobar degeneration.Neuropathology. 2018 Feb;38(1):72-81. doi: 10.1111/neup.12428. Epub 2017 Sep 27. Neuropathology. 2018. PMID: 28960544 Review.
Cited by
-
Targeted depletion of TDP-43 expression in the spinal cord motor neurons leads to the development of amyotrophic lateral sclerosis-like phenotypes in mice.J Biol Chem. 2012 Aug 10;287(33):27335-44. doi: 10.1074/jbc.M112.359000. Epub 2012 Jun 20. J Biol Chem. 2012. PMID: 22718760 Free PMC article.
-
The overexpression of TDP-43 in astrocytes causes neurodegeneration via a PTP1B-mediated inflammatory response.J Neuroinflammation. 2020 Oct 14;17(1):299. doi: 10.1186/s12974-020-01963-6. J Neuroinflammation. 2020. PMID: 33054766 Free PMC article.
-
FTD and ALS--translating mouse studies into clinical trials.Nat Rev Neurol. 2015 Jun;11(6):360-6. doi: 10.1038/nrneurol.2015.65. Epub 2015 May 5. Nat Rev Neurol. 2015. PMID: 25939274 Review.
-
Astrocyte-Neuron Interactions Contributing to Amyotrophic Lateral Sclerosis Progression.Adv Neurobiol. 2024;39:285-318. doi: 10.1007/978-3-031-64839-7_12. Adv Neurobiol. 2024. PMID: 39190080 Review.
-
Phenotypic diversity in ALS and the role of poly-conformational protein misfolding.Acta Neuropathol. 2021 Jul;142(1):41-55. doi: 10.1007/s00401-020-02222-x. Epub 2020 Sep 15. Acta Neuropathol. 2021. PMID: 32930869 Free PMC article. Review.
References
-
- Mackenzie IR, Feldman HH. Ubiquitin immunohistochemistry suggests classic motor neuron disease, motor neuron disease with dementia, and frontotemporal dementia of the motor neuron disease type represent a clinicopathologic spectrum. J Neuropathol Exp Neurol. 2005;64:730–739. - PubMed
-
- Murphy JM, et al. Continuum of frontal lobe impairment in amyotrophic lateral sclerosis. Arch Neurol. 2007;64:530–534. - PubMed
-
- Neumann M, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. - PubMed
-
- Arai T, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

