Neisseria meningitidis GNA2132, a heparin-binding protein that induces protective immunity in humans
- PMID: 20133713
- PMCID: PMC2840514
- DOI: 10.1073/pnas.0915162107
Neisseria meningitidis GNA2132, a heparin-binding protein that induces protective immunity in humans
Abstract
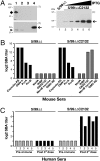
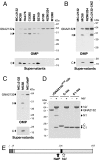
GNA2132 is a Neisseria meningitidis antigen of unknown function, discovered by reverse vaccinology, which has been shown to induce bactericidal antibodies in animal models. Here we show that this antigen induces protective immunity in humans and it is recognized by sera of patients after meningococcal disease. The protein binds heparin in vitro through an Arg-rich region and this property correlates with increased survival of the unencapsulated bacterium in human serum. Furthermore, two proteases, the meningococcal NalP and human lactoferrin, cleave the protein upstream and downstream from the Arg-rich region, respectively. We conclude that GNA2132 is an important protective antigen of N. meningitidis and we propose to rename it, Neisserial Heparin Binding Antigen (NHBA).
Conflict of interest statement
The authors declare no conflict of interest. The sponsor is a full-time employee of Novartis Vaccines and Diagnostics, Siena, Italy.
Figures


References
-
- Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369:2196–2210. - PubMed
-
- Borrow R, et al. Neisseria meningitidis group B correlates of protection and assay standardization—international meeting report Emory University, Atlanta, Georgia, United States, 16-17 March 2005. Vaccine. 2006;24:5093–5107. - PubMed
-
- Stephens DS. Conquering the meningococcus. FEMS Microbiol Rev. 2007;31:3–14. - PubMed
-
- Pizza M, et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science. 2000;287:1816–1820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

