Human and mouse adipose-derived cells support feeder-independent induction of pluripotent stem cells
- PMID: 20133714
- PMCID: PMC2840462
- DOI: 10.1073/pnas.0910172106
Human and mouse adipose-derived cells support feeder-independent induction of pluripotent stem cells
Abstract
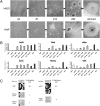
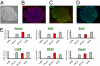
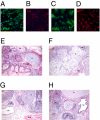
Although adipose tissue is an expandable and readily attainable source of proliferating, multipotent stem cells, its potential for use in regenerative medicine has not been extensively explored. Here we report that adult human and mouse adipose-derived stem cells can be reprogrammed to induced pluripotent stem (iPS) cells with substantially higher efficiencies than those reported for human and mouse fibroblasts. Unexpectedly, both human and mouse iPS cells can be obtained in feeder-free conditions. We discovered that adipose-derived stem cells intrinsically express high levels of pluripotency factors such as basic FGF, TGFbeta, fibronectin, and vitronectin and can serve as feeders for both autologous and heterologous pluripotent cells. These results demonstrate a great potential for adipose-derived cells in regenerative therapeutics and as a model for studying the molecular mechanisms of feeder-free iPS generation and maintenance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–154. - PubMed
-
- Park IH, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. - PubMed
-
- Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

