Delivery of foreign antigens by engineered outer membrane vesicle vaccines
- PMID: 20133740
- PMCID: PMC2840271
- DOI: 10.1073/pnas.0805532107
Delivery of foreign antigens by engineered outer membrane vesicle vaccines
Abstract
As new disease threats arise and existing pathogens grow resistant to conventional interventions, attention increasingly focuses on the development of vaccines to induce protective immune responses. Given their admirable safety records, protein subunit vaccines are attractive for widespread immunization, but their disadvantages include poor immunogenicity and expensive manufacture. We show here that engineered Escherichia coli outer membrane vesicles (OMVs) are an easily purified vaccine-delivery system capable of greatly enhancing the immunogenicity of a low-immunogenicity protein antigen without added adjuvants. Using green-fluorescent protein (GFP) as the model subunit antigen, genetic fusion of GFP with the bacterial hemolysin ClyA resulted in a chimeric protein that elicited strong anti-GFP antibody titers in immunized mice, whereas immunization with GFP alone did not elicit such titers. Harnessing the specific secretion of ClyA to OMVs, the ClyA-GFP fusion was found localized in OMVs, resulting in engineered recombinant OMVs. The anti-GFP humoral response in mice immunized with the engineered OMV formulations was indistinguishable from the response to the purified ClyA-GFP fusion protein alone and equal to purified proteins absorbed to aluminum hydroxide, a standard adjuvant. In a major improvement over current practice, engineered OMVs containing ClyA-GFP were easily isolated by ultracentrifugation, effectively eliminating the need for laborious antigen purification from cell-culture expression systems. With the diverse collection of heterologous proteins that can be functionally localized with OMVs when fused with ClyA, this work signals the possibility of OMVs as a robust and tunable technology platform for a new generation of prophylactic and therapeutic vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

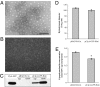
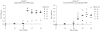

References
-
- Singh M, Chakrapani A, O’Hagan D. Nanoparticles and microparticles as vaccine-delivery systems. Expert Rev Vaccines. 2007;6:797–808. - PubMed
-
- Morein B, Sundquist B, Höglund S, Dalsgaard K, Osterhaus A. Iscom, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. Nature. 1984;308:457–460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

