Molecular mimics can induce a nonautoaggressive repertoire that preempts induction of autoimmunity
- PMID: 20133742
- PMCID: PMC2823870
- DOI: 10.1073/pnas.0914508107
Molecular mimics can induce a nonautoaggressive repertoire that preempts induction of autoimmunity
Abstract
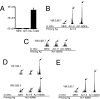
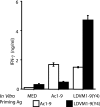
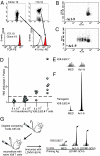
To determine the role that competition plays in a molecular mimic's capacity to induce autoimmunity, we studied the ability of naïve encephalitogenic T cells to expand in response to agonist altered peptide ligands (APLs), some capable of stimulating both self-directed and exclusively APL-specific T cells. Our results show that although the APLs capable of stimulating exclusively APL-specific T cells are able to expand encephalitogenic T cells in vitro, the encephalitogenic repertoire is effectively outcompeted in vivo when the APL is used as the priming immunogen. Competition as a mechanism was supported by: (i) the demonstration of a population of exclusively APL-specific T cells, (ii) an experiment in which an encephalitogenic T cell population was successfully outcompeted by adoptively transferred naïve T cells, and (iii) demonstrating that the elimination of competing T cells bestowed an APL with the ability to expand naïve encephalitogenic T cells in vivo. In total, these experiments support the existence of a reasonably broad T cell repertoire responsive to a molecular mimic (e.g., a microbial agent), of which the exclusively mimic-specific component tends to focus the immune response on the invading pathogen, whereas the rare cross-reactive, potentially autoreactive T cells are often preempted from becoming involved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
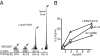

References
-
- Bhardwaj V, Kumar V, Geysen HM, Sercarz EE. Degenerate recognition of a dissimilar antigenic peptide by myelin basic protein-reactive T cells. Implications for thymic education and autoimmunity. J Immunol. 1993;151:5000–5010. - PubMed
-
- Hagerty DT, Allen PM. Intramolecular mimicry. Identification and analysis of two cross-reactive T cell epitopes within a single protein. J Immunol. 1995;155:2993–3001. - PubMed
-
- Loftus C, Huseby E, Gopaul P, Beeson C, Goverman J. Highly cross-reactive T cell responses to myelin basic protein epitopes reveal a nonpredictable form of TCR degeneracy. J Immunol. 1999;162:6451–6457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

