Mutant EGFR is required for maintenance of glioma growth in vivo, and its ablation leads to escape from receptor dependence
- PMID: 20133782
- PMCID: PMC2823874
- DOI: 10.1073/pnas.0914356107
Mutant EGFR is required for maintenance of glioma growth in vivo, and its ablation leads to escape from receptor dependence
Abstract
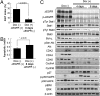
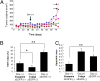
Epidermal growth factor receptor (EGFR) gene amplification is the most common genetic alteration in high-grade glioma, and approximately 50% of EGFR-amplified tumors also harbor a constitutively active mutant form of the receptor, DeltaEGFR. Although DeltaEGFR greatly enhances tumor growth and is thus an attractive target for anti-glioma therapies, recent clinical experiences with EGFR kinase inhibitors have been disappointing, because resistance is common and tumors eventually recur. Interestingly, it has not been established whether DeltaEGFR is required for maintenance of glioma growth in vivo, and, by extension, if it truly represents a rational therapeutic target. Here, we demonstrate that in vivo silencing of regulatable DeltaEGFR with doxycycline attenuates glioma growth and, therefore, that it is crucial for maintenance of enhanced tumorigenicity. Similar to the clinical experience, tumors eventually regained aggressive growth after a period of stasis, but interestingly, without re-expression of DeltaEGFR. To determine how tumors acquired this ability, we found that a unique gene, KLHDC8, herein referred to as SDeltaE (Substitute for DeltaEGFR Expression)-1, is highly expressed in these tumors, which have escaped dependence on DeltaEGFR. SDeltaE-1 is also expressed in human gliomas and knockdown of its expression in DeltaEGFR-independent "escaper" tumors suppressed tumor growth. Taken together, we conclude that DeltaEGFR is required for both glioma establishment and maintenance, and that gliomas undergo selective pressure in vivo to employ alternative compensatory pathways to maintain aggressiveness in the event of EGFR silencing. Such alternative pathways function as substitutes for DeltaEGFR signaling and should therefore be considered as potential targets for additional therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma.Genes Dev. 2010 Aug 15;24(16):1731-45. doi: 10.1101/gad.1890510. Genes Dev. 2010. PMID: 20713517 Free PMC article.
-
EGFRvIII promotes glioma angiogenesis and growth through the NF-κB, interleukin-8 pathway.Oncogene. 2012 Sep 6;31(36):4054-66. doi: 10.1038/onc.2011.563. Epub 2011 Dec 5. Oncogene. 2012. PMID: 22139077 Free PMC article.
-
Growth suppression of intracranial xenografted glioblastomas overexpressing mutant epidermal growth factor receptors by systemic administration of monoclonal antibody (mAb) 806, a novel monoclonal antibody directed to the receptor.Cancer Res. 2001 Jul 15;61(14):5349-54. Cancer Res. 2001. PMID: 11454673
-
Aberrant receptor signaling in human malignant gliomas: mechanisms and therapeutic implications.Cancer Lett. 2001 Jan;162 Suppl:S17-S21. doi: 10.1016/s0304-3835(00)00648-0. Cancer Lett. 2001. PMID: 11164186 Review.
-
The epidermal growth factor receptor in malignant gliomas: pathogenesis and therapeutic implications.Expert Opin Ther Targets. 2007 Apr;11(4):463-72. doi: 10.1517/14728222.11.4.463. Expert Opin Ther Targets. 2007. PMID: 17373877 Review.
Cited by
-
Targeting EGFR for treatment of glioblastoma: molecular basis to overcome resistance.Curr Cancer Drug Targets. 2012 Mar;12(3):197-209. doi: 10.2174/156800912799277557. Curr Cancer Drug Targets. 2012. PMID: 22268382 Free PMC article. Review.
-
Epidermal growth factor receptor as a molecular determinant of glioblastoma response to dopamine receptor D2 inhibitors.Neuro Oncol. 2021 Mar 25;23(3):400-411. doi: 10.1093/neuonc/noaa188. Neuro Oncol. 2021. PMID: 32830856 Free PMC article.
-
EGFR mutation-induced alternative splicing of Max contributes to growth of glycolytic tumors in brain cancer.Cell Metab. 2013 Jun 4;17(6):1000-1008. doi: 10.1016/j.cmet.2013.04.013. Epub 2013 May 23. Cell Metab. 2013. PMID: 23707073 Free PMC article.
-
Charting DENR-dependent translation reinitiation uncovers predictive uORF features and links to circadian timekeeping via Clock.Nucleic Acids Res. 2019 Jun 4;47(10):5193-5209. doi: 10.1093/nar/gkz261. Nucleic Acids Res. 2019. PMID: 30982898 Free PMC article.
-
Cell-Based Glioma Models for Anticancer Drug Screening: From Conventional Adherent Cell Cultures to Tumor-Specific Three-Dimensional Constructs.Cells. 2024 Dec 17;13(24):2085. doi: 10.3390/cells13242085. Cells. 2024. PMID: 39768176 Free PMC article. Review.
References
-
- Libermann TA, et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985;313:144–147. - PubMed
-
- Ekstrand AJ, et al. Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991;51:2164–2172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

