Two physically, functionally, and developmentally distinct peritoneal macrophage subsets
- PMID: 20133793
- PMCID: PMC2823920
- DOI: 10.1073/pnas.0915000107
Two physically, functionally, and developmentally distinct peritoneal macrophage subsets
Abstract
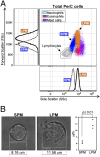
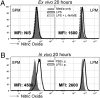
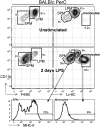
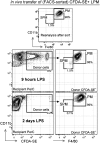
The peritoneal cavity (PerC) is a unique compartment within which a variety of immune cells reside, and from which macrophages (MØ) are commonly drawn for functional studies. Here we define two MØ subsets that coexist in PerC in adult mice. One, provisionally called the large peritoneal MØ (LPM), contains approximately 90% of the PerC MØ in unstimulated animals but disappears rapidly from PerC following lipopolysaccharide (LPS) or thioglycolate stimulation. These cells express high levels of the canonical MØ surface markers, CD11b and F4/80. The second subset, referred to as small peritoneal MØ (SPM), expresses substantially lower levels of CD11b and F4/80 but expresses high levels of MHC-II, which is not expressed on LPM. SPM, which predominates in PerC after LPS or thioglycolate stimulation, does not derive from LPM. Instead, it derives from blood monocytes that rapidly enter the PerC after stimulation and differentiate to mature SPM within 2 to 4 d. Both subsets show clear phagocytic activity and both produce nitric oxide (NO) in response to LPS stimulation in vivo. However, their responses to LPS show key differences: in vitro, LPS stimulates LPM, but not SPM, to produce NO; in vivo, LPS stimulates both subsets to produce NO, albeit with different response patterns. These findings extend current models of MØ heterogeneity and shed new light on PerC MØ diversity, development, and function. Thus, they introduce a new context for interpreting (and reinterpreting) data from ex vivo studies with PerC MØ.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Taylor PR, Brown GD, Geldhof AB, Martinez-Pomares L, Gordon S. Pattern recognition receptors and differentiation antigens define murine myeloid cell heterogeneity ex vivo. Eur J Immunol. 2003;33:2090–2097. - PubMed
-
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. - PubMed
-
- Taylor PR, et al. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. - PubMed
-
- Cohn ZA. The structure and function of monocytes and macrophages. Adv Immunol. 1968;9:163–214. - PubMed
-
- van Furth R. Monocyte production during inflammation. Comp Immunol Microbiol Infect Dis. 1985;8:205–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

