Unexpected role of clathrin adaptor AP-1 in MHC-dependent positive selection of T cells
- PMID: 20133794
- PMCID: PMC2823916
- DOI: 10.1073/pnas.0913671107
Unexpected role of clathrin adaptor AP-1 in MHC-dependent positive selection of T cells
Abstract
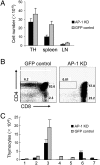
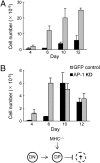
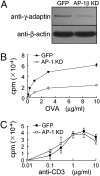
Trafficking of transmembrane receptors to a specific intracellular compartment is conducted by adaptor molecules that bind to target motifs within the cytoplasmic domains of cargo proteins. We generated mice containing a lymphoid-specific deficiency of AP-1 using RNAi knockdown technology. Inhibition of AP-1 expression in thymocytes blocks progression from double-positive immature thymocytes, resulting in complete absence of CD4(+) single-positive thymocytes and severe reduction of CD3(+)CD8(+) single-positive thymocytes. Analysis of the contribution of AP-1 deficiency on the interaction between mature CD4(+) T cells and antigen-presenting cells revealed that AP-1 is essential to efficient immune synapse formation and associated T cell activation, suggesting a possible mechanism of AP-1 function in thymocyte development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
TCR signaling for initiation and completion of thymocyte positive selection has distinct requirements for ligand quality and presenting cell type.J Immunol. 2000 Sep 15;165(6):3015-22. doi: 10.4049/jimmunol.165.6.3015. J Immunol. 2000. PMID: 10975810
-
Gasp, a Grb2-associating protein, is critical for positive selection of thymocytes.Proc Natl Acad Sci U S A. 2009 Sep 22;106(38):16345-50. doi: 10.1073/pnas.0908593106. Epub 2009 Sep 14. Proc Natl Acad Sci U S A. 2009. PMID: 19805304 Free PMC article.
-
Maturation-dependent licensing of naive T cells for rapid TNF production.PLoS One. 2010 Nov 24;5(11):e15038. doi: 10.1371/journal.pone.0015038. PLoS One. 2010. PMID: 21124839 Free PMC article.
-
Antigen-presenting cells and T-lymphocytes homing to the thymus shape T cell development.Immunol Lett. 2018 Dec;204:9-15. doi: 10.1016/j.imlet.2018.10.003. Epub 2018 Oct 9. Immunol Lett. 2018. PMID: 30308217 Review.
-
Roles of autophagy in lymphocytes: reflections and directions.Cell Mol Immunol. 2010 Mar;7(2):104-7. doi: 10.1038/cmi.2009.115. Epub 2010 Feb 1. Cell Mol Immunol. 2010. PMID: 20118969 Free PMC article. Review.
Cited by
-
Leucine zipper transcription factor-like 1 binds adaptor protein complex-1 and 2 and participates in trafficking of transferrin receptor 1.PLoS One. 2020 Jan 2;15(1):e0226298. doi: 10.1371/journal.pone.0226298. eCollection 2020. PLoS One. 2020. PMID: 31895934 Free PMC article.
-
Age-related transcriptional modules and TF-miRNA-mRNA interactions in neonatal and infant human thymus.PLoS One. 2020 Apr 15;15(4):e0227547. doi: 10.1371/journal.pone.0227547. eCollection 2020. PLoS One. 2020. PMID: 32294112 Free PMC article.
-
Deficiency of AP1 Complex Ap1g1 in Zebrafish Model Led to Perturbation of Neurodevelopment, Female and Male Fertility; New Insight to Understand Adaptinopathies.Int J Mol Sci. 2023 Apr 12;24(8):7108. doi: 10.3390/ijms24087108. Int J Mol Sci. 2023. PMID: 37108275 Free PMC article.
-
Role of inositol phospholipid signaling in natural killer cell biology.Front Immunol. 2013 Mar 6;4:47. doi: 10.3389/fimmu.2013.00047. eCollection 2013. Front Immunol. 2013. PMID: 23508471 Free PMC article.
-
Transcriptome and methylome of the supraoptic nucleus provides insights into the age-dependent loss of neuronal plasticity.Front Aging Neurosci. 2023 Aug 30;15:1223273. doi: 10.3389/fnagi.2023.1223273. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37711995 Free PMC article.
References
-
- Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. - PubMed
-
- Werlen G, Hausmann B, Naeher D, Palmer E. Signaling life and death in the thymus: Timing is everything. Science. 2003;299:1859–1863. - PubMed
-
- Aliahmad P, Kaye J. Commitment issues: Linking positive selection signals and lineage diversification in the thymus. Immunol Rev. 2006;209:253–273. - PubMed
-
- Peterson DA, DiPaolo RJ, Kanagawa O, Unanue ER. Cutting edge: Negative selection of immature thymocytes by a few peptide-MHC complexes: Differential sensitivity of immature and mature T cells. J Immunol. 1999;162:3117–3120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

