Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation
- PMID: 20133797
- PMCID: PMC2823886
- DOI: 10.1073/pnas.0915018107
Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation
Abstract
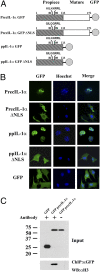
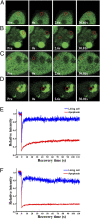
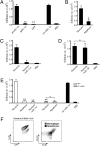
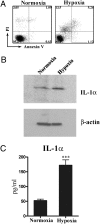
IL-1alpha, like IL-1beta, possesses multiple inflammatory and immune properties. However, unlike IL-1beta, the cytokine is present intracellularly in healthy tissues and is not actively secreted. Rather, IL-1alpha translocates to the nucleus and participates in transcription. Here we show that intracellular IL-1alpha is a chromatin-associated cytokine and highly dynamic in the nucleus of living cells. During apoptosis, IL-1alpha concentrates in dense nuclear foci, which markedly reduces its mobile nature. In apoptotic cells, IL-1alpha is retained within the chromatin fraction and is not released along with the cytoplasmic contents. To simulate the in vivo inflammatory response to cells undergoing different mechanisms of death, lysates of cells were embedded in Matrigel plugs and implanted into mice. Lysates from cells undergoing necrosis recruited cells of the myeloid lineage into the Matrigel, whereas lysates of necrotic cells lacking IL-1alpha failed to recruit an infiltrate. In contrast, lysates of cells undergoing apoptotic death were inactive. Cells infiltrating the Matrigel were due to low concentrations (20-50 pg) of the IL-1alpha precursor containing the receptor interacting C-terminal, whereas the N-terminal propiece containing the nuclear localization site failed to do so. When normal keratinocytes were subjected to hypoxia, the constitutive IL-1alpha precursor was released into the supernatant. Thus, after an ischemic event, the IL-1alpha precursor is released by hypoxic cells and incites an inflammatory response by recruiting myeloid cells into the area. Tissues surrounding the necrotic site also sustain damage from the myeloid cells. Nuclear trafficking and differential release during necrosis vs. apoptosis demonstrate that inflammation by IL-1alpha is tightly controlled.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Apte RN, Voronov E. Is interleukin-1 a good or bad ‘guy’ in tumor immunobiology and immunotherapy? Immunol Rev. 2008;222:222–241. - PubMed
-
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. - PubMed
-
- Mosley B, et al. The interleukin-1 receptor binds the human interleukin-1 alpha precursor but not the interleukin-1 beta precursor. J Biol Chem. 1987;262:2941–2944. - PubMed
-
- Buryskova M, Pospisek M, Grothey A, Simmet T, Burysek L. Intracellular interleukin-1alpha functionally interacts with histone acetyltransferase complexes. J Biol Chem. 2004;279:4017–4026. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

