A role for FKBP52 in Tau protein function
- PMID: 20133804
- PMCID: PMC2823896
- DOI: 10.1073/pnas.0914957107
A role for FKBP52 in Tau protein function
Abstract
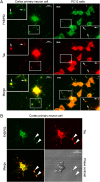
Tau is a microtubule-associated protein, which is widely expressed in the central nervous system, predominantly in neurons, where it regulates microtubule dynamics, axonal transport, and neurite outgrowth. The aberrant assembly of Tau is the hallmark of several human neurodegenerative diseases, collectively known as tauopathies. They include Alzheimer's disease, Pick's disease, progressive supranuclear palsy, and frontotemporal dementia and parkinsonism linked to chromosome 17. Several abnormalities in Tau, such as hyperphosphorylation and aggregation, alter its function and are central to the pathogenic process. Here, we describe biochemical and functional interactions between FKBP52 and Tau. FKBP52 is a member of the FKBP (FK506-binding protein) family that comprises intracellular protein effectors of immunosuppressive drugs (such as FK506 and rapamycin). We found that FKBP52, which is abundant in brain, binds directly and specifically to Tau, especially in its hyperphosphorylated form. The relevance of this observation was confirmed by the colocalization of both proteins in the distal part of the axons of cortical neurons and by the antagonistic effect of FKBP52 on the ability of Tau to promote microtubule assembly. Overexpression of FKBP52 in differentiated PC12 cells prevented the accumulation of Tau and resulted in reduced neurite length. Taken together, these findings indicate a role for FKBP52 in Tau function and may help to decipher and modulate the events involved in Tau-induced neurodegeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

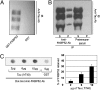
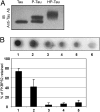

 ) or presence of 1.7 μM (23 μg for HT40) different human Tau isoforms (as indicated in panels A‒F) without FKBP52 (◇) or with 3.5 μM (55 μg) FKBP52 (▲). Tau isoforms differ from each other by the number of repeats in the microtubule-binding domain and insertions in the N terminus. The labeling of Tau isoforms uses the published nomenclature (21). (G) This control experiment was carried out as in A, except that 3.5 μM GST (▲) was used instead of FKBP52.
) or presence of 1.7 μM (23 μg for HT40) different human Tau isoforms (as indicated in panels A‒F) without FKBP52 (◇) or with 3.5 μM (55 μg) FKBP52 (▲). Tau isoforms differ from each other by the number of repeats in the microtubule-binding domain and insertions in the N terminus. The labeling of Tau isoforms uses the published nomenclature (21). (G) This control experiment was carried out as in A, except that 3.5 μM GST (▲) was used instead of FKBP52.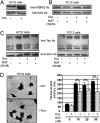
References
-
- Schreiber SL. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991;251:283–287. - PubMed
-
- Steiner JP, et al. High brain densities of the immunophilin FKBP colocalized with calcineurin. Nature. 1992;358:584–587. - PubMed
-
- Snyder SH, Sabatini DM. Immunophilins and the nervous system. Nat Med. 1995;1:32–37. - PubMed
-
- Gold BG. FK506 and the role of immunophilins in nerve regeneration. Mol Neurobiol. 1997;15:285–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

