The CD40-CD40L pathway contributes to the proinflammatory function of intestinal epithelial cells in inflammatory bowel disease
- PMID: 20133813
- PMCID: PMC2843472
- DOI: 10.2353/ajpath.2010.090461
The CD40-CD40L pathway contributes to the proinflammatory function of intestinal epithelial cells in inflammatory bowel disease
Abstract
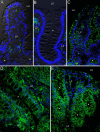
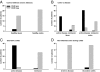
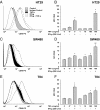
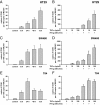
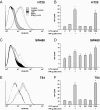
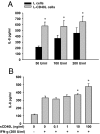
In inflammatory bowel diseases (IBD), intestinal epithelial cells (IECs) are involved in the outbalanced immune responses toward luminal antigens. However, the signals responsible for this proinflammatory capacity of IECs in IBD remain unclear. The CD40/CD40L interaction activates various pathways in immune and nonimmune cells related to inflammation and was shown to be critical for the development of IBD. Here we demonstrate CD40 expression within IECs during active IBD. Endoscopically obtained biopsies taken from Crohn's disease (n = 112) and ulcerative colitis patients (n = 67) consistently showed immunofluorescence staining for CD40 in IECs of inflamed ileal or colonic mucosa. In noninvolved mucosa during active disease, tissue obtained during Crohn's disease or ulcerative colitis in remission and biopsies from healthy controls (n = 38) IECs almost entirely lacked CD40 staining. Flow cytometry and RT-PCR analysis using different intestinal epithelial cell lines (HT29, SW480, and T84) showed IFN-gamma to effectively induce CD40 in IECs. Cells were virtually unresponsive to LPS or whole E. coli regarding CD40 expression. In addition, a moderate induction of CD40 was found in response to TNF-alpha, which exerted synergistical effects with IFN-gamma. CD40 ligation by CD40L-transfected murine fibroblasts or soluble CD40L increased the secretion of IL-8 in IFN-gamma pretreated HT29 cells. Our findings provide evidence for the epithelial expression and modulation of CD40 in IBD-affected mucosa and indicate its involvement in the proinflammatory function of IECs.
Figures
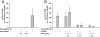
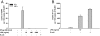

Similar articles
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
MicroRNA 301A Promotes Intestinal Inflammation and Colitis-Associated Cancer Development by Inhibiting BTG1.Gastroenterology. 2017 May;152(6):1434-1448.e15. doi: 10.1053/j.gastro.2017.01.049. Epub 2017 Feb 11. Gastroenterology. 2017. PMID: 28193514
-
Activation of Epithelial Signal Transducer and Activator of Transcription 1 by Interleukin 28 Controls Mucosal Healing in Mice With Colitis and Is Increased in Mucosa of Patients With Inflammatory Bowel Disease.Gastroenterology. 2017 Jul;153(1):123-138.e8. doi: 10.1053/j.gastro.2017.03.015. Epub 2017 Mar 23. Gastroenterology. 2017. PMID: 28342759
-
Critical role of the CD40 CD40-ligand pathway in regulating mucosal inflammation-driven angiogenesis in inflammatory bowel disease.Gut. 2007 Sep;56(9):1248-56. doi: 10.1136/gut.2006.111989. Epub 2007 Feb 22. Gut. 2007. PMID: 17317789 Free PMC article.
-
IL-1 and CD40/CD40L platelet complex: elements of induction of Crohn's disease and new therapeutic targets.Arch Pharm Res. 2021 Jan;44(1):117-132. doi: 10.1007/s12272-020-01296-1. Epub 2021 Jan 4. Arch Pharm Res. 2021. PMID: 33394309 Review.
Cited by
-
The CD40-ATP-P2X 7 Receptor Pathway: Cell to Cell Cross-Talk to Promote Inflammation and Programmed Cell Death of Endothelial Cells.Front Immunol. 2019 Dec 17;10:2958. doi: 10.3389/fimmu.2019.02958. eCollection 2019. Front Immunol. 2019. PMID: 31921199 Free PMC article. Review.
-
Genetically driven target tissue overexpression of CD40: a novel mechanism in autoimmune disease.J Immunol. 2012 Sep 15;189(6):3043-53. doi: 10.4049/jimmunol.1200311. Epub 2012 Aug 10. J Immunol. 2012. PMID: 22888137 Free PMC article.
-
Identification of IL-27 as a novel regulator of major histocompatibility complex class I and class II expression, antigen presentation, and processing in intestinal epithelial cells.Front Immunol. 2023 Sep 25;14:1226809. doi: 10.3389/fimmu.2023.1226809. eCollection 2023. Front Immunol. 2023. PMID: 37818353 Free PMC article.
-
Multipotent role of platelets in inflammatory bowel diseases: a clinical approach.World J Gastroenterol. 2014 Mar 28;20(12):3180-90. doi: 10.3748/wjg.v20.i12.3180. World J Gastroenterol. 2014. PMID: 24696603 Free PMC article. Review.
-
Bioactive indanes: insight into the bioactivity of indane dimers related to the lead anti-inflammatory molecule PH46A.J Pharm Pharmacol. 2020 Jul;72(7):927-937. doi: 10.1111/jphp.13269. Epub 2020 Apr 16. J Pharm Pharmacol. 2020. PMID: 32301120 Free PMC article.
References
-
- Bouma G, Strober W. The immmunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. - PubMed
-
- Hershberg RM, Mayer LF. Antigen processing and presentation by intestinal epithelial cells-polarity and complexity. Immunol Today. 2000;21:123–128. - PubMed
-
- Snoeck V, Goddeeris B, Cox E. The role of enterocytes in the intestinal barrier function and antigen uptake. Microbes Infect. 2005;7:997–1004. - PubMed
-
- Dotan I, Allez M, Nakazawa A, Brimnes J, Schulder-Katz M, Mayer L. Intestinal epithelial cells from inflammatory bowel disease patients preferentially stimulate CD4+ T cells to proliferate and secrete interferon-gamma. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1630–G1640. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

