The alternative splicing repressors hnRNP A1/A2 and PTB influence pyruvate kinase isoform expression and cell metabolism
- PMID: 20133837
- PMCID: PMC2838216
- DOI: 10.1073/pnas.0914845107
The alternative splicing repressors hnRNP A1/A2 and PTB influence pyruvate kinase isoform expression and cell metabolism
Abstract
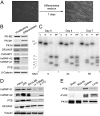
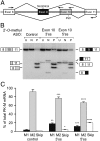
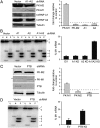
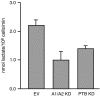
Cancer cells preferentially metabolize glucose by aerobic glycolysis, characterized by increased lactate production. This distinctive metabolism involves expression of the embryonic M2 isozyme of pyruvate kinase, in contrast to the M1 isozyme normally expressed in differentiated cells, and it confers a proliferative advantage to tumor cells. The M1 and M2 pyruvate-kinase isozymes are expressed from a single gene through alternative splicing of a pair of mutually exclusive exons. We measured the expression of M1 and M2 mRNA and protein isoforms in mouse tissues, tumor cell lines, and during terminal differentiation of muscle cells, and show that alternative splicing regulation is sufficient to account for the levels of expressed protein isoforms. We further show that the M1-specific exon is actively repressed in cancer-cell lines--although some M1 mRNA is expressed in cell lines derived from brain tumors--and demonstrate that the related splicing repressors hnRNP A1 and A2, as well as the polypyrimidine-tract-binding protein PTB, contribute to this control. Downregulation of these splicing repressors in cancer-cell lines using shRNAs rescues M1 isoform expression and decreases the extent of lactate production. These findings extend the links between alternative splicing and cancer, and begin to define some of the factors responsible for the switch to aerobic glycolysis.
Conflict of interest statement
The authors declare a conflict of interest. L.C.C. and M.G.V.H. are associated with Agios Pharmaceuticals.
Figures

References
-
- Christofk HR, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. - PubMed
-
- Dombrauckas JD, Santarsiero BD, Mesecar AD. Structural basis for tumor pyruvate kinase M2 allosteric regulation and catalysis. Biochemistry. 2005;44:9417–9429. - PubMed
-
- Noguchi T, Yamada K, Inoue H, Matsuda T, Tanaka T. The L- and R-type isozymes of rat pyruvate kinase are produced from a single gene by use of different promoters. J Biol Chem. 1987;262:14366–14371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

