Rapid evolution of stability and productivity at the origin of a microbial mutualism
- PMID: 20133857
- PMCID: PMC2836651
- DOI: 10.1073/pnas.0908456107
Rapid evolution of stability and productivity at the origin of a microbial mutualism
Abstract
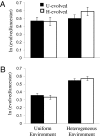
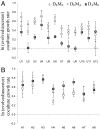
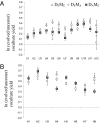
Mutualistic interactions are taxonomically and functionally diverse. Despite their ubiquity, however, the basic ecological and evolutionary processes underlying their origin and maintenance are poorly understood. A major reason for this is the lack of an experimentally tractable model system. We examine the evolution of an experimentally imposed obligate mutualism between sulfate-reducing and methanogenic microorganisms that have no known history of previous interaction. Twenty-four independent pairings (cocultures) of the bacterium Desulfovibrio vulgaris and the archaeon Methanococcus maripaludis were established and followed for 300 community doublings in two environments, one allowing for the development of a heterogeneous distribution of resources and the other not. Evolved cocultures grew up to 80% faster and were up to 30% more productive (biomass yield per mole of substrate) than the ancestors. The evolutionary process was marked by periods of significant instability leading to extinction of two of the cocultures, but it resulted in more stable, efficient, and productive mutualisms for most replicated pairings. Comparisons of evolved cocultures with those assembled from one evolved mutualist and one ancestral mutualist showed that evolution of both species contributed to improved productivity. Surprisingly, however, overall improvements in growth rate and yield were less than the sum of the individual contributions, suggesting antagonistic interactions between mutations from the coevolved populations. Physical constraints on the transfer of metabolites in the evolution environment affected the evolution of M. maripaludis, but not of D. vulgaris. Together, these results demonstrate that challenges can imperil nascent obligate mutualisms and demonstrate the evolutionary responses that enable their persistence and future evolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sachs JL, Simms EL. Pathways to mutualism breakdown. Trends Ecol Evol. 2006;21:585–592. - PubMed
-
- Bergstrom CT, et al. In: Genetic and Cultural Evolution of Cooperation. Hammerstein P, editor. Cambridge, MA: MIT Press; 2003. pp. 241–256.
-
- Sachs JL, Mueller UG, Wilcox TP, Bull JJ. The evolution of cooperation. Q Rev Biol. 2004;79:135–160. - PubMed
-
- May RM. In: Theoretical Ecology: Principles and Applications. May RM, editor. Philadelphia: WB Saunders; 1976. pp. 49–71.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

