Accelerated carcinogenesis following liver regeneration is associated with chronic inflammation-induced double-strand DNA breaks
- PMID: 20133864
- PMCID: PMC2836653
- DOI: 10.1073/pnas.0908867107
Accelerated carcinogenesis following liver regeneration is associated with chronic inflammation-induced double-strand DNA breaks
Abstract
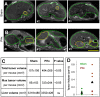
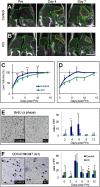
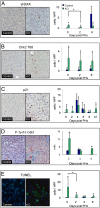
Hepatocellular carcinoma (HCC) is the third leading cause of cancer mortality worldwide and is considered to be the outcome of chronic liver inflammation. Currently, the main treatment for HCC is surgical resection. However, survival rates are suboptimal partially because of tumor recurrence in the remaining liver. Our aim was to understand the molecular mechanisms linking liver regeneration under chronic inflammation to hepatic tumorigenesis. Mdr2-KO mice, a model of inflammation-associated cancer, underwent partial hepatectomy (PHx), which led to enhanced hepatocarcinogenesis. Moreover, liver regeneration in these mice was severely attenuated. We demonstrate the activation of the DNA damage-response machinery and increased genomic instability during early liver inflammatory stages resulting in hepatocyte apoptosis, cell-cycle arrest, and senescence and suggest their involvement in tumor growth acceleration subsequent to PHx. We propose that under the regenerative proliferative stress induced by liver resection, the genomic unstable hepatocytes generated during chronic inflammation escape senescence and apoptosis and reenter the cell cycle, triggering the enhanced tumorigenesis. Thus, we clarify the immediate and long-term contributions of the DNA damage response to HCC development and recurrence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Karin M, Greten FR. NF-kappaB: Linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. - PubMed
-
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. - PubMed
-
- Kundu JK, Surh YJ. Inflammation: Gearing the journey to cancer. Mutat Res. 2008;659:15–30. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

