Functional reciprocity between Na+ channel Nav1.6 and beta1 subunits in the coordinated regulation of excitability and neurite outgrowth
- PMID: 20133873
- PMCID: PMC2836661
- DOI: 10.1073/pnas.0909434107
Functional reciprocity between Na+ channel Nav1.6 and beta1 subunits in the coordinated regulation of excitability and neurite outgrowth
Abstract
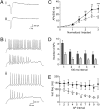
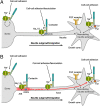
Voltage-gated Na(+) channel (VGSC) beta1 subunits regulate cell-cell adhesion and channel activity in vitro. We previously showed that beta1 promotes neurite outgrowth in cerebellar granule neurons (CGNs) via homophilic cell adhesion, fyn kinase, and contactin. Here we demonstrate that beta1-mediated neurite outgrowth requires Na(+) current (I(Na)) mediated by Na(v)1.6. In addition, beta1 is required for high-frequency action potential firing. Transient I(Na) is unchanged in Scn1b (beta1) null CGNs; however, the resurgent I(Na), thought to underlie high-frequency firing in Na(v)1.6-expressing cerebellar neurons, is reduced. The proportion of axon initial segments (AIS) expressing Na(v)1.6 is reduced in Scn1b null cerebellar neurons. In place of Na(v)1.6 at the AIS, we observed an increase in Na(v)1.1, whereas Na(v)1.2 was unchanged. This indicates that beta1 is required for normal localization of Na(v)1.6 at the AIS during the postnatal developmental switch to Na(v)1.6-mediated high-frequency firing. In agreement with this, beta1 is normally expressed with alpha subunits at the AIS of P14 CGNs. We propose reciprocity of function between beta1 and Na(v)1.6 such that beta1-mediated neurite outgrowth requires Na(v)1.6-mediated I(Na), and Na(v)1.6 localization and consequent high-frequency firing require beta1. We conclude that VGSC subunits function in macromolecular signaling complexes regulating both neuronal excitability and migration during cerebellar development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Catterall WA. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. - PubMed
-
- Hille B. Ionic Channels of Excitable Membranes. 2nd Ed. Sunderland, MA: Sinauer Associates; 1992.
-
- Goldin AL, et al. Nomenclature of voltage-gated sodium channels. Neuron. 2000;28:365–368. - PubMed
-
- Trimmer JS, Rhodes KJ. Localization of voltage-gated ion channels in mammalian brain. Annu Rev Physiol. 2004;66:477–519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

