Synthetic amyloid-beta oligomers impair long-term memory independently of cellular prion protein
- PMID: 20133875
- PMCID: PMC2836680
- DOI: 10.1073/pnas.0911829107
Synthetic amyloid-beta oligomers impair long-term memory independently of cellular prion protein
Abstract
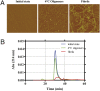
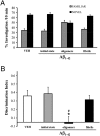
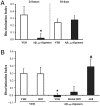
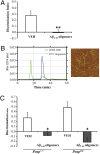
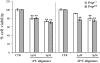
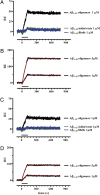
Inability to form new memories is an early clinical sign of Alzheimer's disease (AD). There is ample evidence that the amyloid-beta (Abeta) peptide plays a key role in the pathogenesis of this disorder. Soluble, bio-derived oligomers of Abeta are proposed as the key mediators of synaptic and cognitive dysfunction, but more tractable models of Abeta-mediated cognitive impairment are needed. Here we report that, in mice, acute intracerebroventricular injections of synthetic Abeta(1-42) oligomers impaired consolidation of the long-term recognition memory, whereas mature Abeta(1-42) fibrils and freshly dissolved peptide did not. The deficit induced by oligomers was reversible and was prevented by an anti-Abeta antibody. It has been suggested that the cellular prion protein (PrP(C)) mediates the impairment of synaptic plasticity induced by Abeta. We confirmed that Abeta(1-42) oligomers interact with PrP(C), with nanomolar affinity. However, PrP-expressing and PrP knock-out mice were equally susceptible to this impairment. These data suggest that Abeta(1-42) oligomers are responsible for cognitive impairment in AD and that PrP(C) is not required.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- McLean CA, et al. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999;46:860–866. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

