Activation of the ROCK1 branch of the transforming growth factor-beta pathway contributes to RAGE-dependent acceleration of atherosclerosis in diabetic ApoE-null mice
- PMID: 20133903
- PMCID: PMC2848909
- DOI: 10.1161/CIRCRESAHA.109.201103
Activation of the ROCK1 branch of the transforming growth factor-beta pathway contributes to RAGE-dependent acceleration of atherosclerosis in diabetic ApoE-null mice
Abstract
Rationale: The multiligand RAGE (receptor for advanced glycation end products) contributes to atherosclerosis in apolipoprotein (Apo)E-null mice.
Objective: To delineate the specific mechanisms by which RAGE accelerated atherosclerosis, we performed Affymetrix gene expression arrays on aortas of nondiabetic and diabetic ApoE-null mice expressing RAGE or devoid of RAGE at nine weeks of age, as this reflected a time point at which frank atherosclerotic lesions were not yet present, but that we would be able to identify the genes likely involved in diabetes- and RAGE-dependent atherogenesis.
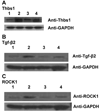
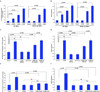
Methods and results: We report that there is very little overlap of the genes that are differentially expressed both in the onset of diabetes in ApoE-null mice, and in the effect of RAGE deletion in diabetic ApoE-null mice. Pathway-Express analysis revealed that the transforming growth factor-beta pathway and focal adhesion pathways might be expected to play a significant role in both the mechanism by which diabetes facilitates the formation of atherosclerotic plaques in ApoE-null mice, and the mechanism by which deletion of RAGE ameliorates this effect. Quantitative polymerase chain reaction studies, Western blotting, and confocal microscopy in aortic tissue and in primary cultures of murine aortic smooth muscle cells supported these findings.
Conclusions: Taken together, our work suggests that RAGE-dependent acceleration of atherosclerosis in ApoE-null mice is dependent, at least in part, on the action of the ROCK1 (rho-associated protein kinase 1) branch of the transforming growth factor-beta pathway.
Figures



References
-
- Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4:1025–1031. - PubMed
-
- Kislinger T, Tanji N, Wendt T, Qu W, Lu Y, Ferran LJ, Jr, Taguchi A, Olson K, Bucciarelli L, Goova M, Hofmann MA, Cataldegirmen G, D'Agati V, Pischetsrieder M, Stern DM, Schmidt AM. Receptor for advanced glycation endproducts mediates inflammation and enhanced expression of tissue factor in vasculature of diabetic apolipoprotein E null mice. Arterioscler Thromb Vasc Biol. 2001;21:905–910. - PubMed
-
- Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla E, Rong LL, Goova MT, Moser B, Kislinger T, Lee DC, Kashypa Y, Stern DM, Schmidt AM. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E null mice. Circ. 2002;106:2827–2835. - PubMed
-
- Wendt T, Harja E, Bucciarelli L, Qu W, Lu Y, Rong LL, Jenkins DG, Stein G, Schmidt AM, Yan SF. RAGE modulates vascular inflammation and atherosclerosis in a murine model of type 2 diabetes. Atherosclerosis. 2006;185:70–77. - PubMed
-
- Harja E, Bu DX, Hudson BI, Chang JS, Shen X, Hallam K, Kalea AZ, Lu Y, Rosario RH, Oruganti S, Nikolla Z, Belov D, Lalla E, Ramasamy R, Yan SF, Schmidt AM. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE −/− mice. J Clin Invest. 2008;118:183–194. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

