Ric-8B stabilizes the alpha subunit of stimulatory G protein by inhibiting its ubiquitination
- PMID: 20133939
- PMCID: PMC2856988
- DOI: 10.1074/jbc.M109.063313
Ric-8B stabilizes the alpha subunit of stimulatory G protein by inhibiting its ubiquitination
Abstract
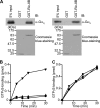
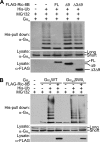
The alpha subunit of stimulatory G protein (G alpha(s)) activates adenylyl cyclase, which catalyzes cAMP production, and regulates many physiological aspects, such as cardiac regulation and endocrine systems. Ric-8B (resistance to inhibitors of cholinesterase 8B) has been identified as the G alpha(s)-binding protein; however, its role in G(s) signaling remains obscure. In this study, we present evidence that Ric-8B specifically and positively regulates G(s) signaling by stabilizing the G alpha(s) protein. An in vitro biochemical study suggested that Ric-8B does not possess guanine nucleotide exchange factor activity. However, knockdown of Ric-8B attenuated beta-adrenergic agonist-induced cAMP accumulation, indicating that Ric-8B positively regulates G(s) signaling. Interestingly, overexpression and knockdown of Ric-8B resulted in an increase and a decrease in the G alpha(s) protein, respectively, without affecting the G alpha(s) mRNA level. We found that the G alpha(s) protein is ubiquitinated and that this ubiquitination is inhibited by Ric-8B. This Ric-8B-mediated inhibition of G alpha(s) ubiquitination requires interaction between Ric-8B and G alpha(s) because Ric-8B splicing variants, which are defective for G alpha(s) binding, failed to inhibit the ubiquitination. Taken together, these results suggest that Ric-8B plays a critical and specific role in the control of G alpha(s) protein levels by modulating G alpha(s) ubiquitination and positively regulates G(s) signaling.
Figures



References
-
- Gilman A. G. (1987) Annu. Rev. Biochem. 56, 615–649 - PubMed
-
- Kaziro Y., Itoh H., Kozasa T., Nakafuku M., Satoh T. (1991) Annu. Rev. Biochem. 60, 349–400 - PubMed
-
- Ross E. M., Wilkie T. M. (2000) Annu. Rev. Biochem. 69, 795–827 - PubMed
-
- Blumer J. B., Cismowski M. J., Sato M., Lanier S. M. (2005) Trends Pharmacol. Sci. 26, 470–476 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

