Insulin-like growth factors are more effective than progastrin in reversing proapoptotic effects of curcumin: critical role of p38MAPK
- PMID: 20133951
- PMCID: PMC2853304
- DOI: 10.1152/ajpgi.00497.2009
Insulin-like growth factors are more effective than progastrin in reversing proapoptotic effects of curcumin: critical role of p38MAPK
Abstract
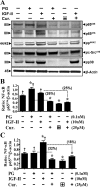
Progastrin and insulin-like growth factors (IGFs) stimulate hyperproliferation of intestinal epithelial cells (IECs) via endocrine/paracrine routes; hyperproliferation is a known risk factor for colon carcinogenesis. In the present study, inhibitory potency of curcumin in the presence or absence of progastrin and/or IGF-II was examined. Progastrin and IGF-II significantly increased proliferation of an immortalized IEC cell line, IEC-18, whereas curcumin decreased the proliferation in a dose-dependent manner. IGF-II was significantly more effective than progastrin in reversing antiproliferative effects of curcumin and reversed proapoptotic effects of curcumin by >80%; progastrin was relatively ineffective toward reversing proapoptotic effects of curcumin. IEC-18 clones were generated to overexpress either progastrin (IEC-PG) or hIGF-II (IEC-IGF). Proliferation of IEC-PG and IEC-IGF clones was increased, compared with that of control clones. Curcumin significantly reduced proliferation of IEC-PG, but not IEC-IGF, clones. Similarly, a human colon cancer cell line, Caco-2 (which expresses autocrine IGF-II), was relatively resistant to inhibitory effects of curcumin. However, Caco-2 cells treated with anti-IGF-II-antibodies were rendered sensitive to inhibitory effects of curcumin. Significant differences in inhibitory potency of curcumin against PG- vs. IGF-II-stimulated growth of IEC-18 cells were not reflected by differences in curcumin-mediated inhibition of activated (phosphorylated) ERKs/IKK(alpha/beta)/p65NF-kappaB and c-Src in wild-type (wt)IEC-18 cells, in response to the two growth factors. Surprisingly, curcumin was almost ineffective in reducing IGF-II-stimulated activation of p38MAPK but significantly reduced progastrin-stimulated phosphorylation of p38. Treatment with a p38MAPK inhibitor resulted in loss of protective effects of IGF-II against inhibitory effects of curcumin. These novel findings suggest that growth factor profile of patients and tumors may dictate inhibitory potency of curcumin and that combination of curcumin + p38MAPK inhibitor may be required for reducing hyperproliferative or tumorigenic response of IECs to endocrine and autocrine IGFs.
Figures





Similar articles
-
Annexin A2 mediates up-regulation of NF-κB, β-catenin, and stem cell in response to progastrin in mice and HEK-293 cells.Gastroenterology. 2011 Feb;140(2):583-595.e4. doi: 10.1053/j.gastro.2010.08.054. Epub 2010 Sep 6. Gastroenterology. 2011. PMID: 20826156 Free PMC article.
-
Antiapoptotic effects of progastrin on pancreatic cancer cells are mediated by sustained activation of nuclear factor-{kappa}B.Cancer Res. 2007 Aug 1;67(15):7266-74. doi: 10.1158/0008-5472.CAN-07-1206. Cancer Res. 2007. PMID: 17671195
-
pp60c-Src Kinase mediates growth effects of the full-length precursor progastrin1-80 peptide on rat intestinal epithelial cells, in vitro.Endocrinology. 2003 Jan;144(1):201-11. doi: 10.1210/en.2002-220501. Endocrinology. 2003. PMID: 12488346
-
Precursor peptide progastrin(1-80) reduces apoptosis of intestinal epithelial cells and upregulates cytochrome c oxidase Vb levels and synthesis of ATP.Am J Physiol Gastrointest Liver Physiol. 2003 Dec;285(6):G1097-110. doi: 10.1152/ajpgi.00216.2003. Epub 2003 Jul 24. Am J Physiol Gastrointest Liver Physiol. 2003. PMID: 12881229
-
Role of Annexin-II in GI cancers: interaction with gastrins/progastrins.Cancer Lett. 2007 Jul 8;252(1):19-35. doi: 10.1016/j.canlet.2006.11.012. Epub 2006 Dec 22. Cancer Lett. 2007. PMID: 17188424 Free PMC article. Review.
Cited by
-
Progastrin Peptides Increase the Risk of Developing Colonic Tumors: Impact on Colonic Stem Cells.Curr Colorectal Cancer Rep. 2012 Dec;8(4):277-289. doi: 10.1007/s11888-012-0144-3. Curr Colorectal Cancer Rep. 2012. PMID: 23226720 Free PMC article.
-
Ophiopogonin D inhibits cell proliferation and induces apoptosis of human laryngocarcinoma through downregulation of cyclin B1 and MMP-9 and upregulation of p38-MAPK signaling.Oncol Lett. 2019 Feb;17(2):1877-1882. doi: 10.3892/ol.2018.9788. Epub 2018 Dec 3. Oncol Lett. 2019. PMID: 30675250 Free PMC article.
-
Epigenetic regulation of human DCLK-1 gene during colon-carcinogenesis: clinical and mechanistic implications.Stem Cell Investig. 2016 Sep 28;3:51. doi: 10.21037/sci.2016.09.07. eCollection 2016. Stem Cell Investig. 2016. PMID: 27777940 Free PMC article.
-
Annexin A2 mediates up-regulation of NF-κB, β-catenin, and stem cell in response to progastrin in mice and HEK-293 cells.Gastroenterology. 2011 Feb;140(2):583-595.e4. doi: 10.1053/j.gastro.2010.08.054. Epub 2010 Sep 6. Gastroenterology. 2011. PMID: 20826156 Free PMC article.
-
Curcumin promotes autophagic survival of a subset of colon cancer stem cells, which are ablated by DCLK1-siRNA.Cancer Res. 2014 May 1;74(9):2487-98. doi: 10.1158/0008-5472.CAN-13-3536. Epub 2014 Mar 13. Cancer Res. 2014. PMID: 24626093 Free PMC article.
References
-
- Bordonaro M, Mariadason JM, Aslam F, Heerdt BG, Augenlicht LH. Butyrate-induced apoptotic cascade in colonic carcinoma cells: modulation of the beta-catenin-Tcf pathway and concordance with effects of sulindac and trichostatin A, but not curcumin. Cell Growth Differ 10: 713–720, 1999 - PubMed
-
- Ciccotosto GD, McLeish A, Hardy KJ, Shulkes A. Expression, processing, and secretion of gastrin in patients with colorectal carcinoma. Gastroenterology 109: 1142–1153, 1995 - PubMed
-
- Cobb S, Wood T, Ceci J, Varro A, Velasco M, Singh P. Intestinal expression of progastrin significantly increases colon carcinogenesis in transgenic mice in response to AOM. Cancer 100: 1311–1323, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

