Cooperation between HNF-1alpha, Cdx2, and GATA-4 in initiating an enterocytic differentiation program in a normal human intestinal epithelial progenitor cell line
- PMID: 20133952
- PMCID: PMC2907224
- DOI: 10.1152/ajpgi.00265.2009
Cooperation between HNF-1alpha, Cdx2, and GATA-4 in initiating an enterocytic differentiation program in a normal human intestinal epithelial progenitor cell line
Abstract
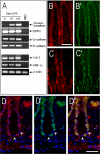
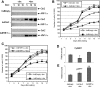
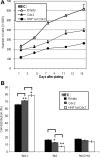
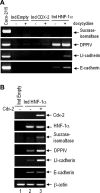
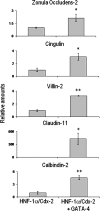
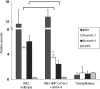
In the intestinal epithelium, the Cdx, GATA, and HNF transcription factor families are responsible for the expression of differentiation markers such as sucrase-isomaltase. Although previous studies have shown that Cdx2 can induce differentiation in rat intestinal IEC-6 cells, no data are available concerning the direct implication of transcription factors on differentiation in human normal intestinal epithelial cell types. We investigated the role of Cdx2, GATA-4, and HNF-1alpha using the undifferentiated human intestinal epithelial crypt cell line HIEC. These transcription factors were tested on proliferation and expression of polarization and differentiation markers. Ectopic expression of Cdx2 or HNF-1alpha, alone or in combination, altered cell proliferation abilities through the regulation of cyclin D1 and p27 expression. HNF-1alpha and GATA-4 together induced morphological modifications of the cells toward polarization, resulting in the appearance of functional features such as microvilli. HNF-1alpha was also sufficient to induce the expression of cadherins and dipeptidylpeptidase, whereas in combination with Cdx2 it allowed the expression of the late differentiation marker sucrase-isomaltase. Large-scale analysis of gene expression confirmed the cooperative effect of these factors. Finally, although DcamKL1 and Musashi-1 expression were downregulated in differentiated HIEC, other intestinal stem cell markers, such as Bmi1, were unaffected. These observations show that, in cooperation with Cdx2, HNF-1alpha acts as a key factor on human intestinal cells to trigger the onset of their functional differentiation program whereas GATA-4 appears to promote morphological changes.
Figures



Similar articles
-
Differential expression of claudin-2 along the human intestine: Implication of GATA-4 in the maintenance of claudin-2 in differentiating cells.J Cell Physiol. 2005 Apr;203(1):15-26. doi: 10.1002/jcp.20189. J Cell Physiol. 2005. PMID: 15389642
-
Hepatocyte nuclear factor-1 alpha, GATA-4, and caudal related homeodomain protein Cdx2 interact functionally to modulate intestinal gene transcription. Implication for the developmental regulation of the sucrase-isomaltase gene.J Biol Chem. 2002 Aug 30;277(35):31909-17. doi: 10.1074/jbc.M204622200. Epub 2002 Jun 11. J Biol Chem. 2002. PMID: 12060663
-
The effect of cytostatic drug treatment on intestine-specific transcription factors Cdx2, GATA-4 and HNF-1alpha in mice.Cancer Chemother Pharmacol. 2006 Jun;57(6):801-10. doi: 10.1007/s00280-005-0119-z. Epub 2005 Sep 15. Cancer Chemother Pharmacol. 2006. PMID: 16163540
-
Role of GATA factors in development, differentiation, and homeostasis of the small intestinal epithelium.Am J Physiol Gastrointest Liver Physiol. 2014 Mar;306(6):G474-90. doi: 10.1152/ajpgi.00119.2013. Epub 2014 Jan 16. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 24436352 Free PMC article. Review.
-
Defining hierarchies of stemness in the intestine: evidence from biomarkers and regulatory pathways.Am J Physiol Gastrointest Liver Physiol. 2014 Aug 1;307(3):G260-73. doi: 10.1152/ajpgi.00066.2014. Epub 2014 Jun 12. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 24924746 Free PMC article. Review.
Cited by
-
Epithelial de-differentiation triggered by co-ordinate epigenetic inactivation of the EHF and CDX1 transcription factors drives colorectal cancer progression.Cell Death Differ. 2022 Nov;29(11):2288-2302. doi: 10.1038/s41418-022-01016-w. Epub 2022 May 23. Cell Death Differ. 2022. PMID: 35606410 Free PMC article.
-
Autophagy is active in normal colon mucosa.Autophagy. 2012 Jun;8(6):893-902. doi: 10.4161/auto.19738. Epub 2012 Jun 1. Autophagy. 2012. PMID: 22652752 Free PMC article.
-
Pharmacological targeting of Sam68 functions in colorectal cancer stem cells.iScience. 2021 Nov 14;24(12):103442. doi: 10.1016/j.isci.2021.103442. eCollection 2021 Dec 17. iScience. 2021. PMID: 34877499 Free PMC article.
-
The H2A.Z histone variant integrates Wnt signaling in intestinal epithelial homeostasis.Nat Commun. 2019 Apr 23;10(1):1827. doi: 10.1038/s41467-019-09899-z. Nat Commun. 2019. PMID: 31015444 Free PMC article.
-
P63 Deficiency and CDX2 Overexpression Lead to Barrett's-Like Metaplasia in Mouse Esophageal Epithelium.Dig Dis Sci. 2021 Dec;66(12):4263-4273. doi: 10.1007/s10620-020-06756-8. Epub 2021 Jan 19. Dig Dis Sci. 2021. PMID: 33469811 Free PMC article.
References
-
- Auclair BA, Benoit YD, Rivard N, Mishina Y, Perreault N. Bone morphogenetic protein signaling is essential for terminal differentiation of the intestinal secretory cell lineage. Gastroenterology 133: 887–896, 2007 - PubMed
-
- Babeu JP, Darsigny M, Lussier CR, Boudreau F. Hepatocyte nuclear factor 4α contributes to an intestinal epithelial phenotype in vitro and plays a partial role in mouse intestinal epithelium differentiation. Am J Physiol Gastrointest Liver Physiol 297: G124–G134, 2009. - PubMed
-
- Babyatsky MW, Podolsky DK. Growth and development of the gastrointestinal tract. In: Textbook of Gastroenterology, edited by Yamada T.Philadelphia, PA: Lippincott, 1999, p. 547–584
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007 - PubMed
-
- Basora N, Herring-Gillam FE, Boudreau F, Perreault N, Pageot LP, Simoneau M, Bouatrouss Y, Beaulieu JF. Expression of functionally distinct variants of the beta(4)A integrin subunit in relation to the differentiation state in human intestinal cells. J Biol Chem 274: 29819–29825, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

