Effectiveness of combined modality radiotherapy of orthotopic human squamous cell carcinomas in Nu/Nu mice using cetuximab, tirapazamine and MnSOD-plasmid liposome gene therapy
- PMID: 20133969
- PMCID: PMC2899489
Effectiveness of combined modality radiotherapy of orthotopic human squamous cell carcinomas in Nu/Nu mice using cetuximab, tirapazamine and MnSOD-plasmid liposome gene therapy
Abstract
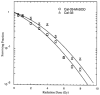
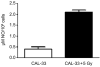
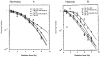
Hypoxic regions limit the radiocontrollability of head and neck carcinomas. Whether or not combinations of plasmid/liposome mediated overexpression of normal tissue protective manganese superoxide dismutase (MnSOD), cetuximab (C225), and the hypoxic cytotoxin tirapazamine (TPZ) enhanced radiotherapeutic effects was tested in a CAL-33 orthotopic mouse cheek tumor model. The tumor volume continued to increase in the control (untreated) mice, with a ninefold increase by 10 days when the tumors exceeded 2 cm(3). The mice receiving 14 Gy only showed reduced tumor growth to 3.1+/-0.1 fold at day 10. The mice receiving MnSOD-PL, C225, TPZ plus 14 Gy had the best outcome with 0.7+/-0.1 fold increase in tumor volume by 10 days (p=0.015) compared to irradiation only. The addition of MnSOD-PL, TPZ, and C225 to irradiation optimized the therapeutic ratio for the local control of hypoxic region-containing CAL-33 orthotopic tumors.
Figures





Similar articles
-
Effect of EGFR antagonists gefitinib (Iressa) and C225 (Cetuximab) on MnSOD-plasmid liposome transgene radiosensitization of a murine squamous cell carcinoma cell line.In Vivo. 2006 Nov-Dec;20(6B):791-6. In Vivo. 2006. PMID: 17203769
-
Effects of MnSOD-plasmid liposome gene therapy on antioxidant levels in irradiated murine oral cavity orthotopic tumors.Radiat Res. 2007 Mar;167(3):289-97. doi: 10.1667/RR0761.1. Radiat Res. 2007. PMID: 17316075
-
Usefulness of combined treatment with mild temperature hyperthermia and/or tirapazamine in the treatment of solid tumors: its independence of p53 status.Cancer Sci. 2003 Jan;94(1):125-33. doi: 10.1111/j.1349-7006.2003.tb01363.x. Cancer Sci. 2003. PMID: 12708486 Free PMC article.
-
Epidermal growth factor receptors as a target for cancer treatment: the emerging role of IMC-C225 in the treatment of lung and head and neck cancers.Semin Oncol. 2002 Feb;29(1 Suppl 4):27-36. doi: 10.1053/sonc.2002.31525. Semin Oncol. 2002. PMID: 11894011 Review.
-
Cetuximab: a review of its use in squamous cell carcinoma of the head and neck and metastatic colorectal cancer.Drugs. 2007;67(17):2585-607. doi: 10.2165/00003495-200767170-00008. Drugs. 2007. PMID: 18034592 Review.
Cited by
-
Nanoparticle-based targeted therapeutics in head-and-neck cancer.Int J Med Sci. 2015 Jan 12;12(2):187-200. doi: 10.7150/ijms.10083. eCollection 2015. Int J Med Sci. 2015. PMID: 25589895 Free PMC article. Review.
-
Esophageal radioprotection by swallowed JP4-039/F15 in thoracic-irradiated mice with transgenic lung tumors.In Vivo. 2014 Jul-Aug;28(4):435-40. In Vivo. 2014. PMID: 24982207 Free PMC article.
-
Antioxidant Approaches to Management of Ionizing Irradiation Injury.Antioxidants (Basel). 2015 Jan 23;4(1):82-101. doi: 10.3390/antiox4010082. Antioxidants (Basel). 2015. PMID: 26785339 Free PMC article.
-
Strategies for discovery of small molecule radiation protectors and radiation mitigators.Front Oncol. 2012 Jan 13;1:59. doi: 10.3389/fonc.2011.00059. eCollection 2011. Front Oncol. 2012. PMID: 22655254 Free PMC article.
-
Avasopasem manganese synergizes with hypofractionated radiation to ablate tumors through the generation of hydrogen peroxide.Sci Transl Med. 2021 May 12;13(593):eabb3768. doi: 10.1126/scitranslmed.abb3768. Sci Transl Med. 2021. PMID: 33980575 Free PMC article.
References
-
- Agarwala SS, Cano E, Heron DE, Johnson J, Myers E, Sandulache V, Bahri S, Ferris R, Wang Y, Argiris A. Long-term outcomes with concurrent carboplatin, paclitaxel and radiation therapy for locally advanced, inoperable head and neck cancer. Annals of Oncology. 2007;18:1224–1229. - PubMed
-
- Le QT, Taira A, Budenz S, et al. Mature results from a randomized phase II trial of cisplatin plus 5-fluorouracil and radiotherapy with or without tirapazamine in patients with resectable stage IV head and neck squamous cell carcinomas. Cancer. 2006;106:1940–1949. - PubMed
-
- Kam MKM, Leung S-F, Zee B, Chau RMC, Suen JJS, Mo F, Lai M, Ho R, Cheung K-Y, Yu BKH, Chiu SKW, Choi PHK, Teo PML, Kwan W-H, Chan ATC. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25(31):4873–4879. - PubMed
-
- Eisbruch A. Reducing xerostomia by IMRT: What may, and may not, be achieved. J Clin Oncol. 2007;25(31):4863–4864. - PubMed
-
- Yao M, Karnell LH, Funk GF, Lu H, Dornfeld K, Buatti JM. Health-related quality-of-life outcomes following IMRT versus conventional radiotherapy for oropharyngeal squamous cell carcinoma. Int J Rad Oncol Biol Phys. 2007;69(5):1354–1360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
