Voltage-gated proton channels find their dream job managing the respiratory burst in phagocytes
- PMID: 20134026
- PMCID: PMC3023998
- DOI: 10.1152/physiol.00039.2009
Voltage-gated proton channels find their dream job managing the respiratory burst in phagocytes
Abstract
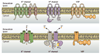
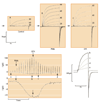
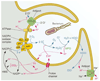
The voltage-gated proton channel bears surprising resemblance to the voltage-sensing domain (S1-S4) of other voltage-gated ion channels but is a dimer with two conduction pathways. The proton channel seems designed for efficient proton extrusion from cells. In phagocytes, it facilitates the production of reactive oxygen species by NADPH oxidase.
Figures
References
-
- Ädelroth P, Brzezinski P. Surface-mediated proton-transfer reactions in membrane-bound proteins. Biochim Biophys Acta. 2004;1655:102–115. - PubMed
-
- Aggarwal SK, MacKinnon R. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron. 1996;16:1169–1177. - PubMed
-
- Åkerfeldt KS, Lear JD, Wasserman ZR, Chung LA, DeGrado WF. Synthetic peptides as models for ion channel proteins. Acc Chem Res. 1993;26:191–197.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

